Centinel Spine
Cervical disc prosthesis to restore disc height and maintain segmental motion. prodisc C Vivo is intended to replace a diseased and/or degenerated intervertebral disc of the cervical spine in patients with symptomatic cervical disc disease. The prodisc C Vivo procedure is intended to significantly reduce pain by allowing for the removal of the diseased disc while restoring disc height and providing the potential for motion at the affected vertebral segment.


- Simple technique with two main steps: trial and implant insertion
- Convex superior plate for ana tomical fixation
- Trapezoidal footprint design for optimal anatomical fit and maximum endplate coverage
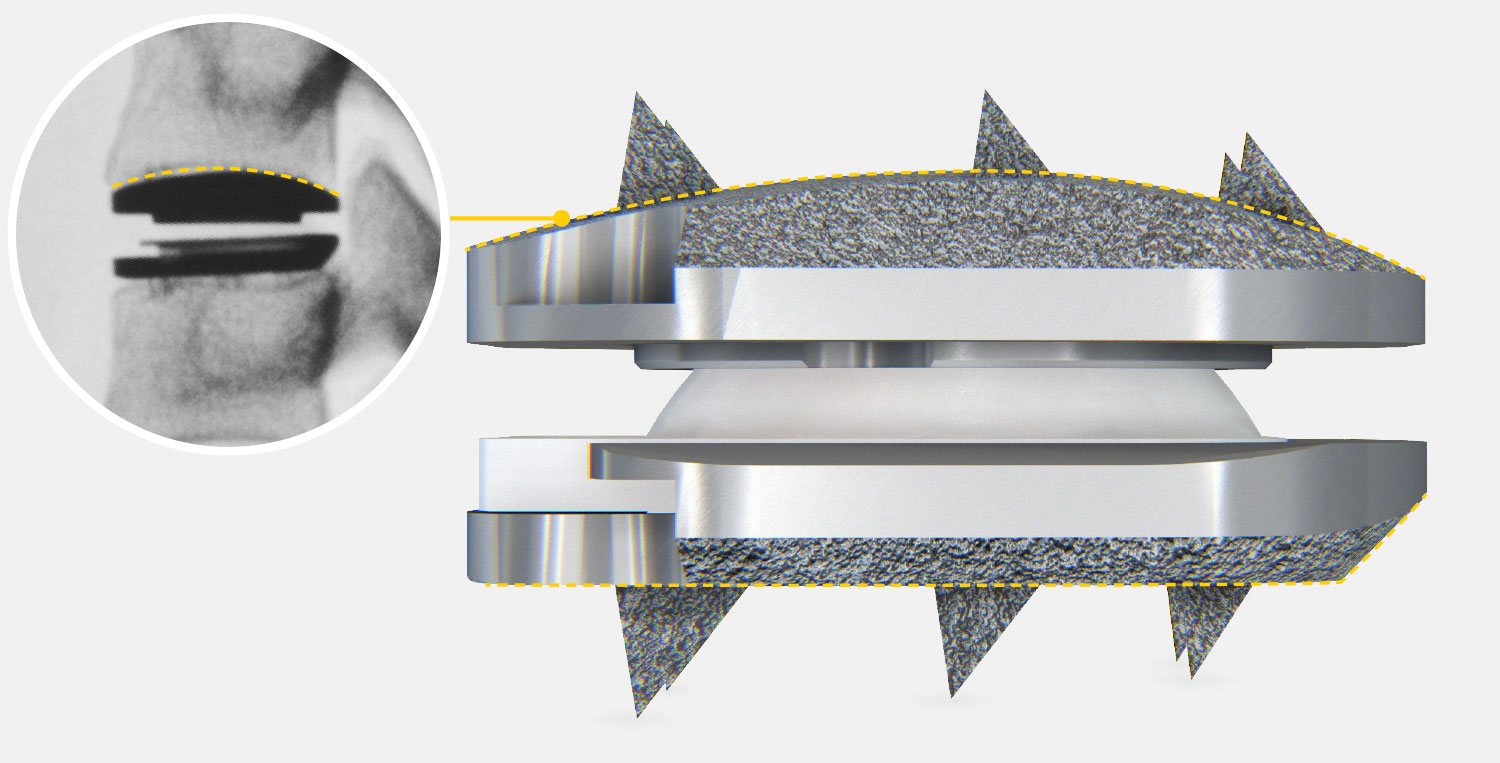
- Superior and inferior implant plmade from titanium alloy for improved MRI compatibility.
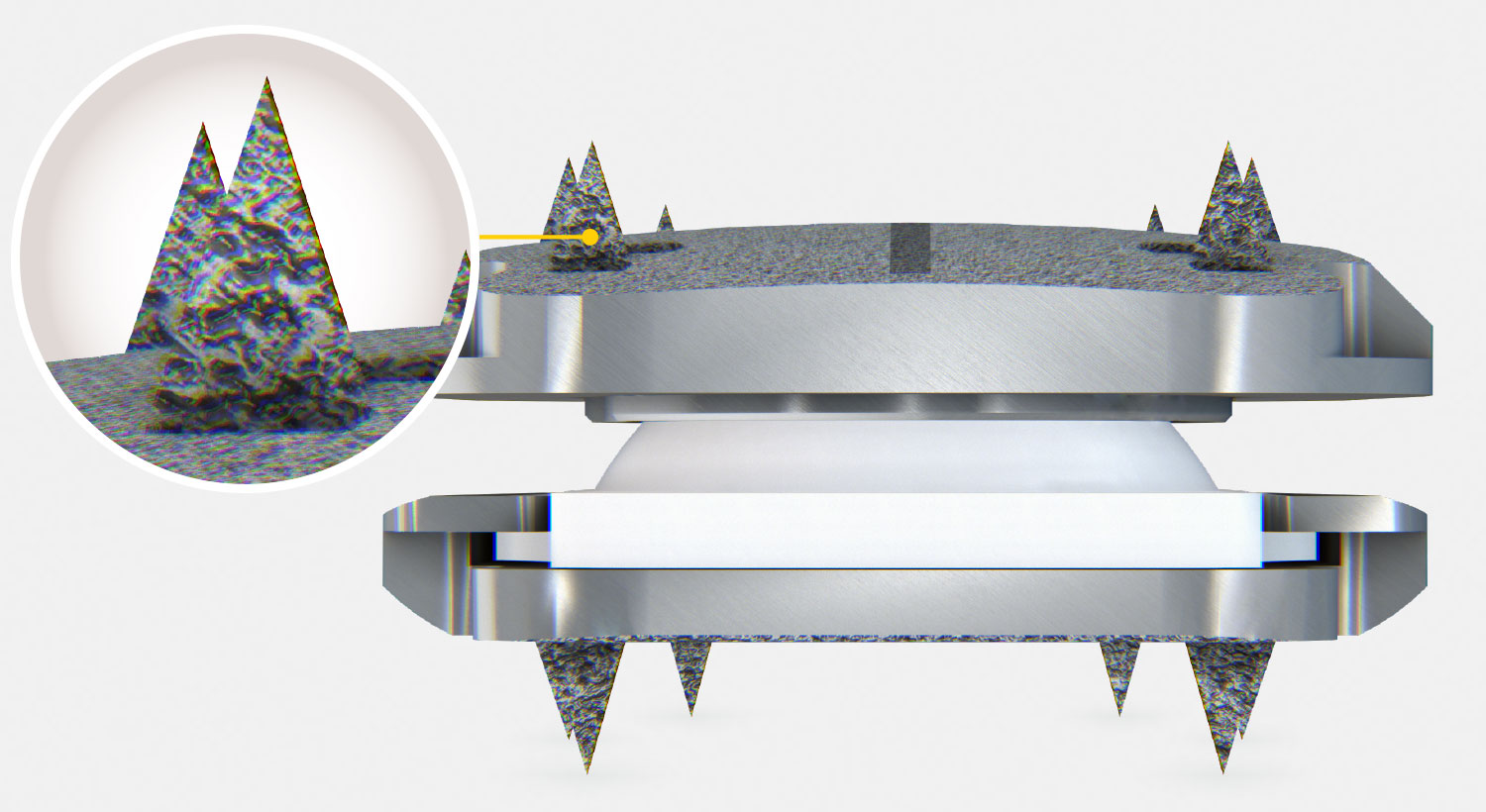
- Rough surface coating of pure titanium allows bony ongrowth
- Inlay made from ultra-high molecular weight polyethylene (UHMWPE)
- UHMWPE on CoCrMo alloy articulation
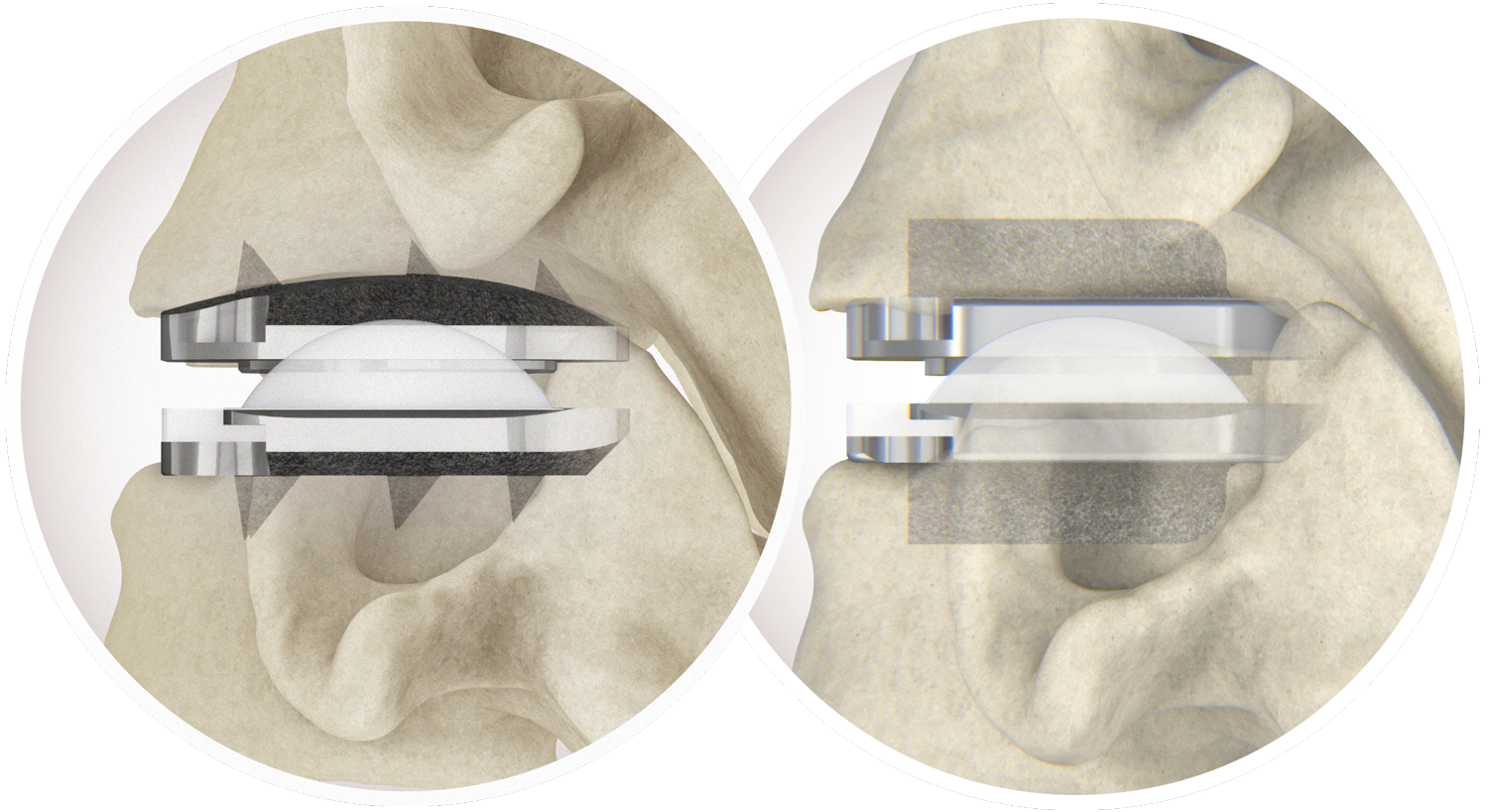
- Permits a physiological range of motion in flexion/extension, rotation, and lateral bending
- Allows for restoration of anatomical balance
- Resists shear forces
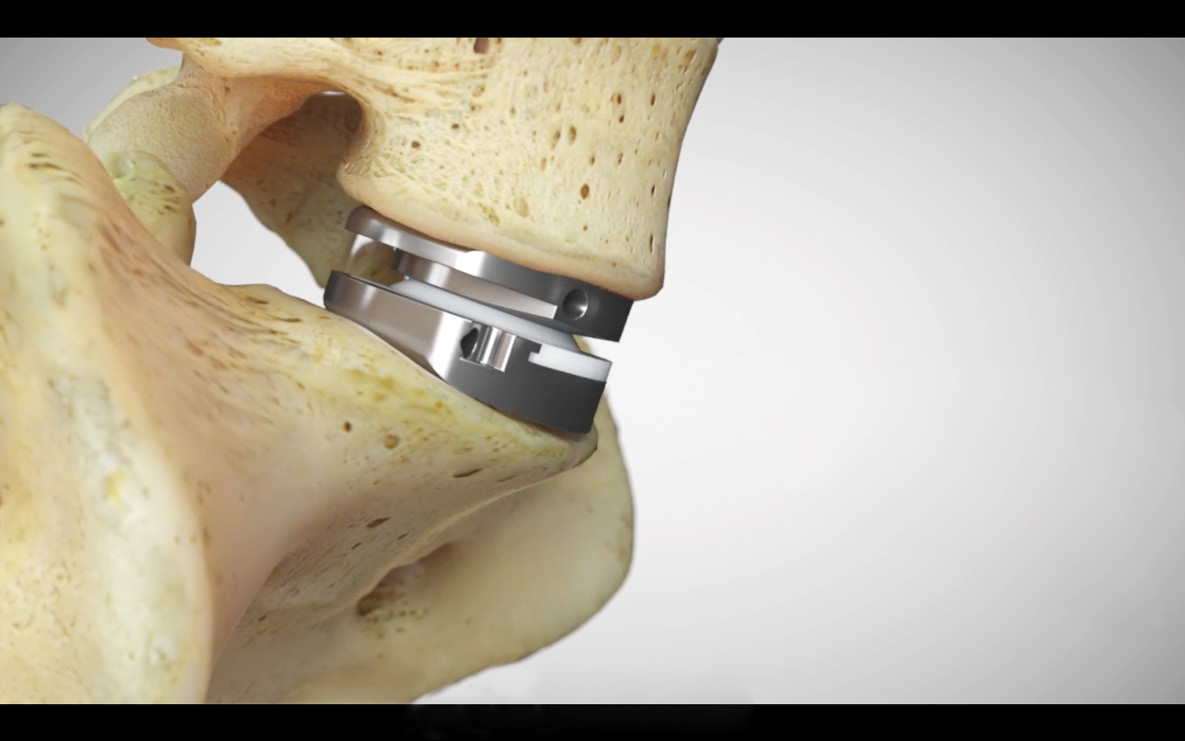
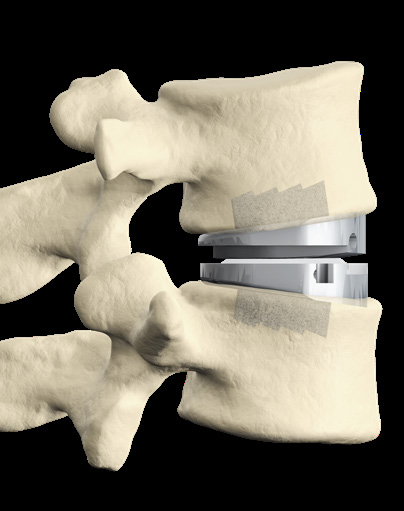
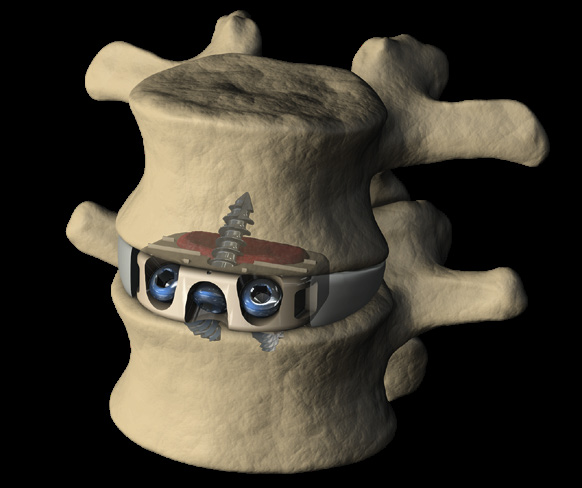
- prodisc C Vivo implants are used to replace a cervical intervertebral disc, to restore disc height and maintain segmental motion.
- The prodisc C Vivo implant consists of two titanium endplates. The superior endplate has a convex shape, while the inferior plate is flat. There are six spikes on both plates.
Inferior Angled Endplates prodisc L was originally developed with angles in both the inferior and superior endplates, to allow optimization of implant choice for each level. The prodisc L Total Disc Replacement system now has a greater selection of endplate angles available, expanding the treatment options. Centinel Spine now manufactures the prodisc L product line including the Inferior Angled Endplates for both international and US surgeons' use. The angle in the inferior plate horizontalizes the segment, reducing the sacral slope and minimizing shear forces on implant. The angle in inferior plate also elevates the lumbosacral joint higher within the pelvis, optimizing lateral bending and axial rotation, which may improve overall motion. The prodisc design provides controlled flexion extension while, at the same time, re-stabilizing the diseased segment. The design incorporates the clinically proven ball and socket concept used in joint replacement implants for over 40 years. With a fixed center of rotation, prodisc provides coupled motion—translation with flexion/extension—which provides stability for the segment while allowing controlled motion and protecting the facet complex against shear forces. Pure translation, in the absence of flexion/extension, may lead to loading and accelerated degeneration of the facet joints at the index and adjacent levels. The prodisc L Total Disc Replacement system now has a greater selection of endplate angles available, expanding the treatment options.

- The prodisc ball and socket provides controlled and predictable coupled motion while protecting the facets from impingement.
- The prodisc fixed core is designed to limit the risks of core expulsion and core locking as can be seen with mobile core implants.
- The prodisc ball and socket restores stability to the motion segment.
- Proven materials used successfully in hip and knee joint replacement implants for over 40 years.
- Patented midline keel provides immediate stability and up to 30% more surface area to enhance the potential for bony on-growth
- The keel promotes predictable, reproducible midline implant placement
- The plasma-sprayed titanium surface on bone contacting surfaces promotes integration and increases stability
- Safe & Reproducible Surgical Technique
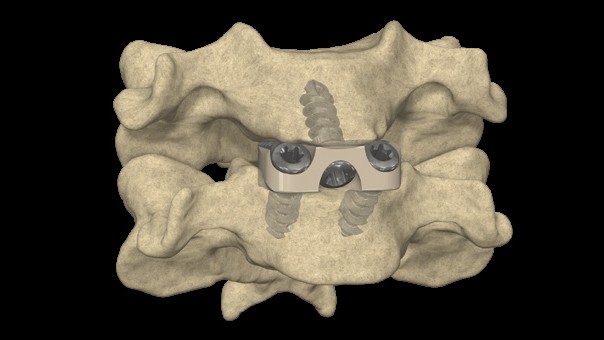
The STALIF technology platform provided the world's first stand-alone (aka Integrated Interbody) device and changed the way spinal fusion surgery is done. Now, a large percentage of spinal fusion procedures take place with stand-alone devices, which provide advantages over alternatives. STALIF enables surgeons to treat diseased spinal segments with a single procedure, enabling faster recovery and return to daily activities. STALIF introduced to the world the idea of providing stability to the spine with a selffixating spacer that enables pinched nerves to be off-loaded. Over 30 years after the first cases with the STALIF technology platform, many spine companies offer 'STALIF-like' devices, and STALIF has evolved as well through 7 technology generations. The STALIF technology platform has been used clinically for over 30 years and is currently in its 7th generation. Each iteration has brought design advancements intended to increase opportunities for fusion or speed patient recovery. STALIF is designed to provide stability between adjacent bones in the spine and provide height to off-load pinched nerves. STALIF is secured with specially designed screws that stabilize the adjacent bones so that a fusion can take place between the two adjacent vertebral bodies. STALIF has been consistently and repeatedly improved to optimize fusion and patient recovery. Features have been added to the device to simplify implantation, provide easier fixation, and minimize incision size without compromising stability. Now in the 7th generation, STALIF provides optimal surgical results and remains many generations ahead of alternative devices.

Flexible: Proprietary lattice structure with a stiffness similar to bone. Lucent: Porous radiolucent sections designed to reduce imaging artifacts. MatriX: FUSE-THRU trabecular scaffold, bioengineered to allow the potential for bony in-growth, ongrowth, and thru-growth. In the quest to improve patient’s quality of life, spine surgeons often struggle to identify clinically validated technologies with state-of-the-art materials, 3D-Printed FLX portfolio solves this by:Providing surgeons with a wide range of solutions, Leveraging the clinically proven STALIF design, Offering design innovations achievable only through advanced additive manufacturing



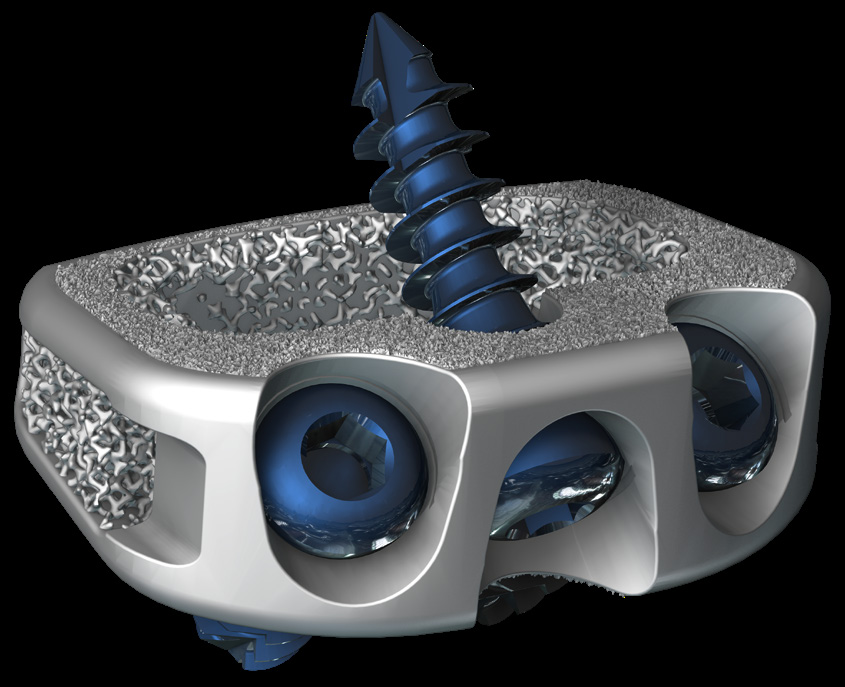



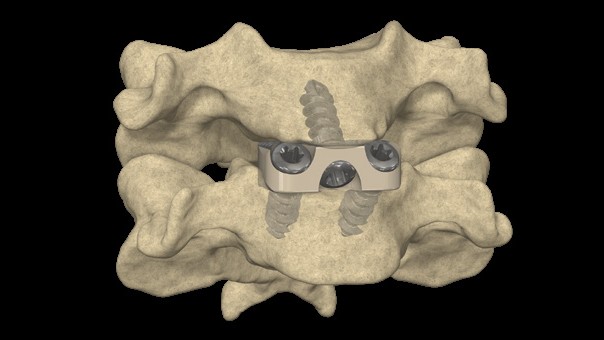
- Eliminates profile of plate, allowing for an optimal load transfer within the vertebral segment
- Match patient anatomy for optimal endplate contact
- Compresses endplates to cage & graft providing optimized segmental loading to support bone development in line with Wolff's Law
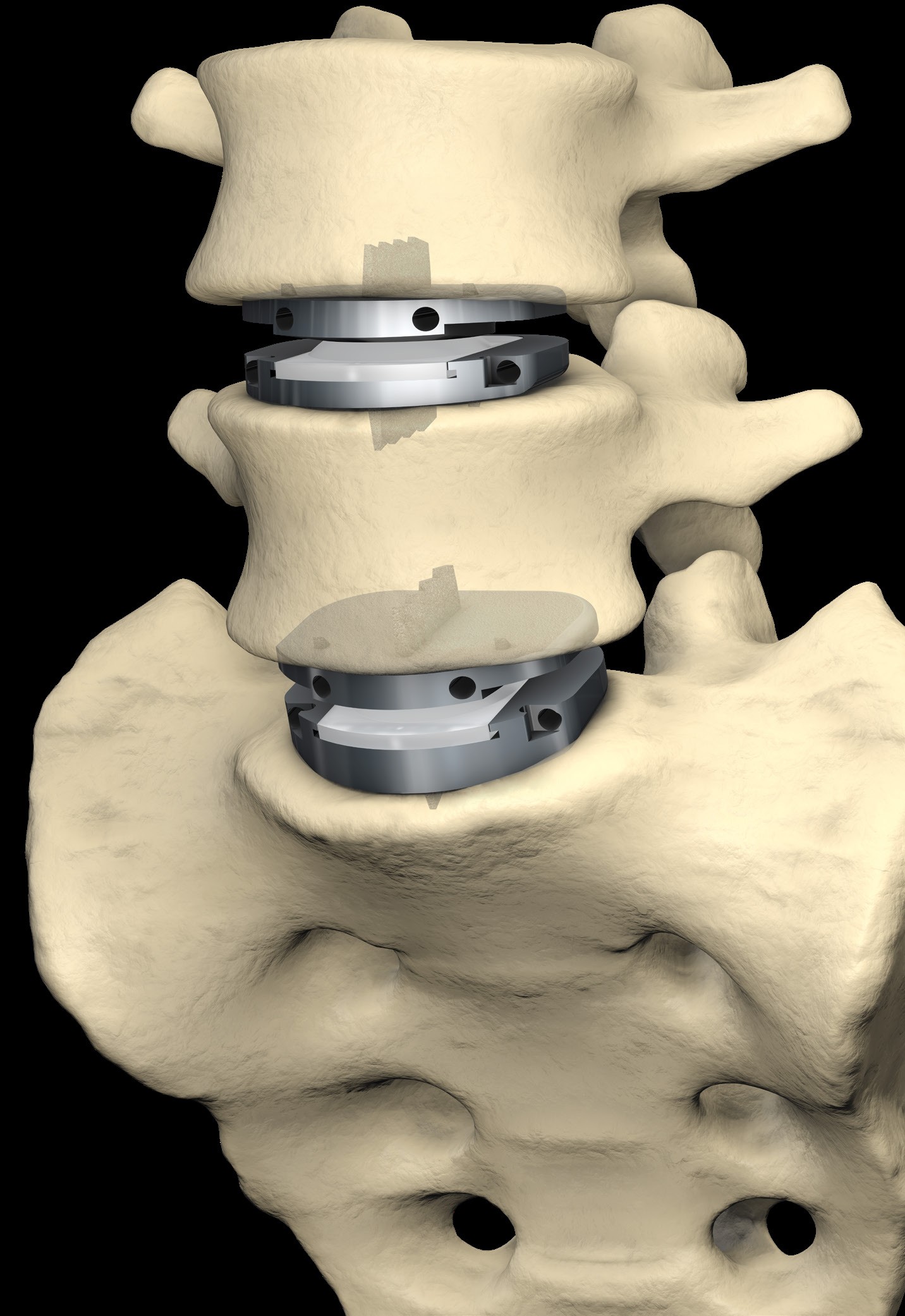
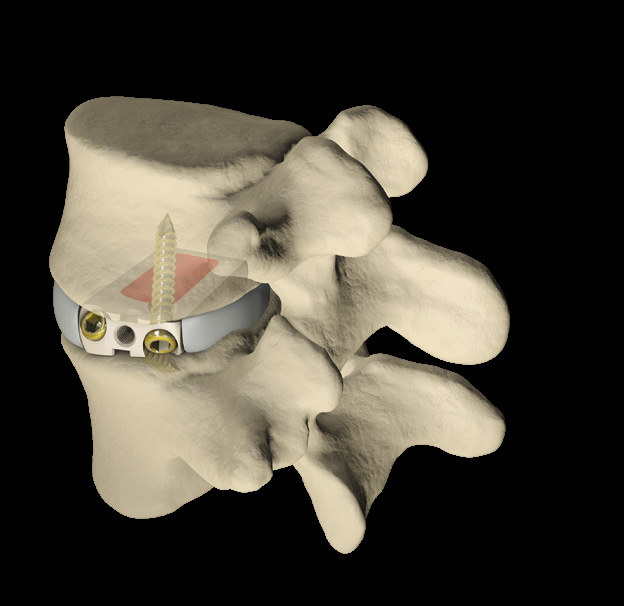
The STALIF C Portfolio is a comprehensive set of instruments and implants designed to support an anterior approach to the cervical spine. The STALIF C portfolio No-Profile® implant designs are engineered to conform to patient anatomy simplify surgery, enhance stability, and maximize opportunities for fusion. STALIF C portfolio Integrated Interbody anterior cervical fusion devices are designed to achieve immediate load-sharing and segmental stability. Innovative implant design complemented by unique cancellous screws co-function during the healing process to form an “integrated” fusion construct that is highly stable. Laboratory evaluations have repeatedly shown that the STALIF C product family attains equivalent biomechanical performance to anterior plate and cage technologies. STALIF C-Ti is an innovative, titanium-surfaced, PEEK Integrated Interbody device design that blends the benefits of titanium and PEEK integrated interbody devices. PEEK has superior visualization properties and a modulus of elasticity that is similar to that of cortical bone while titanium is cell-friendly and enables human mesenchymal stem cells to adhere to the surface and proliferate. The Ti-ACTIVE surface topography is designed to provide enhanced cellular attachment and proliferation vs rougher titanium surfaces, enhancing opportunities for fusion.
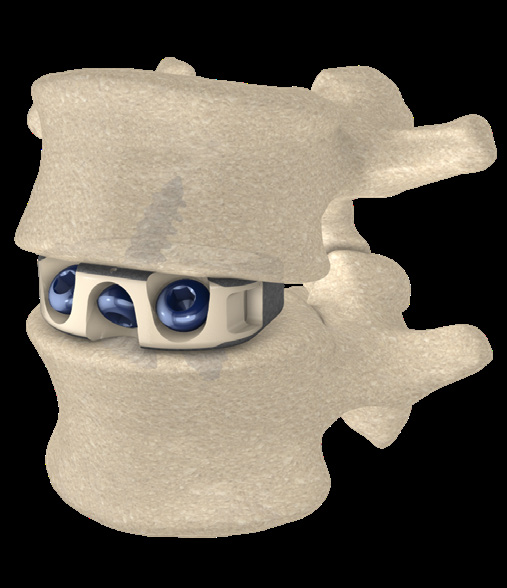

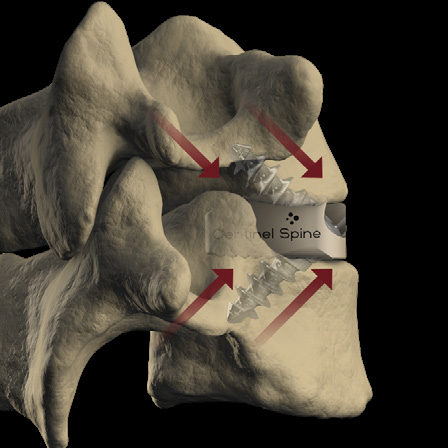
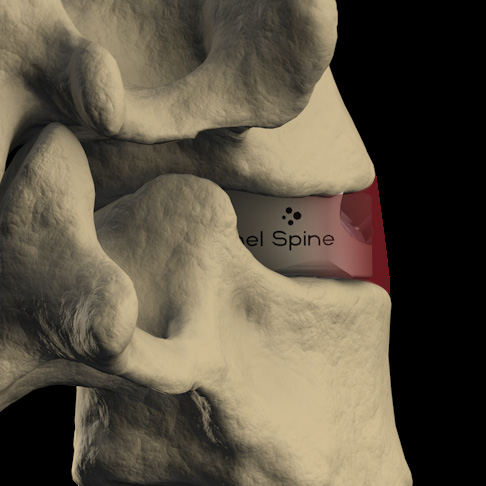


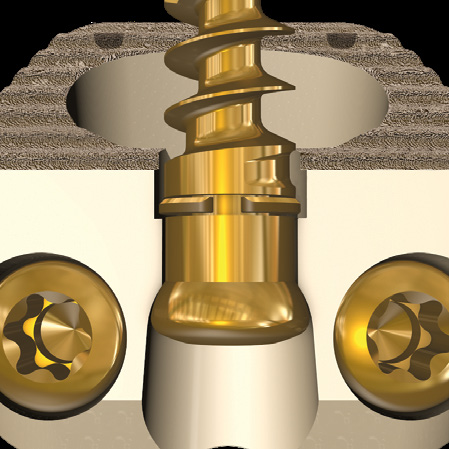
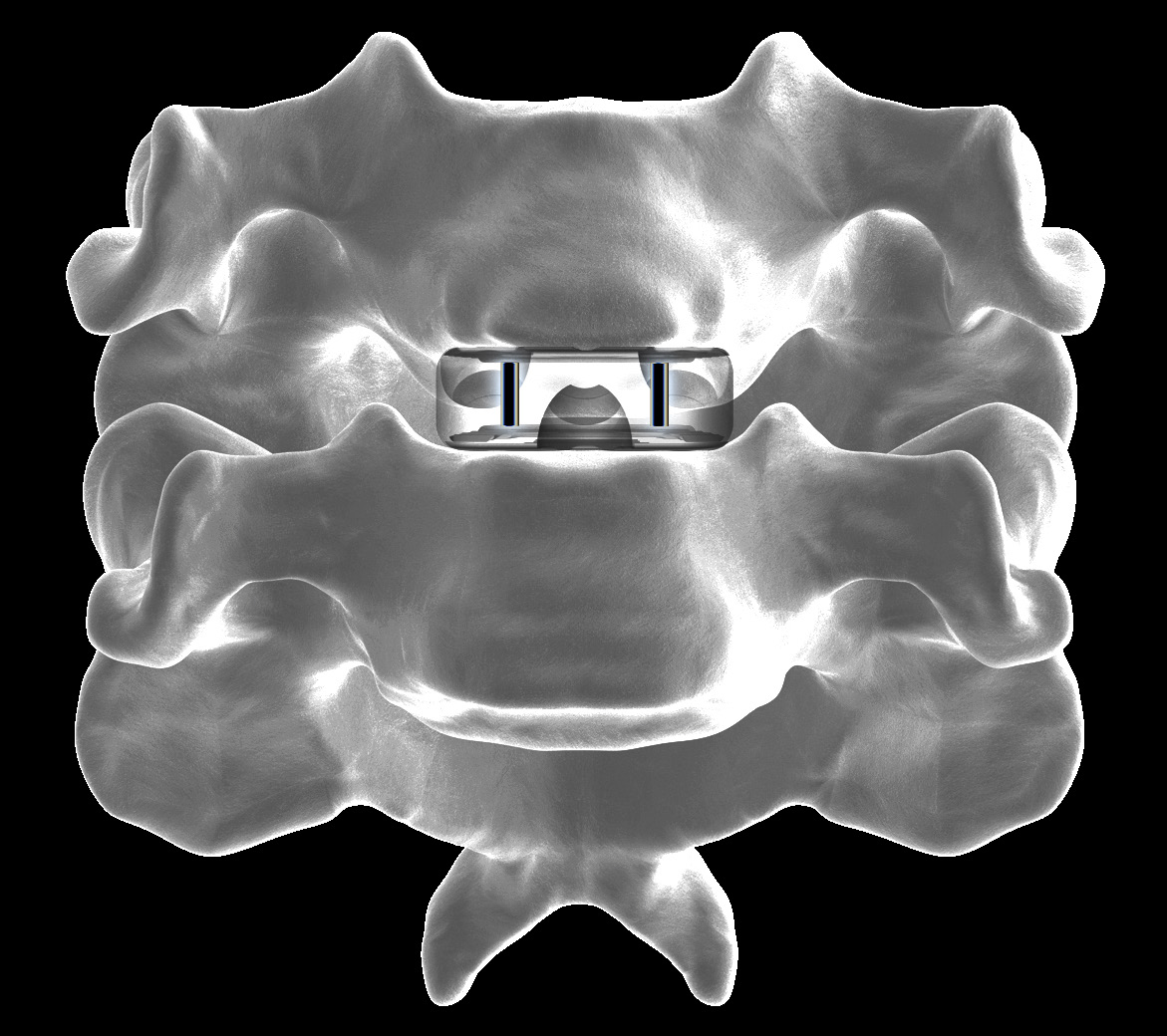
- 3-screw design utilizes a midline convergence pattern in conjunction with an optimal horizontal inclination.
- Screw trajectories are intended to reduce incidence of backout and increase expulsion resistance.
- Titanium split ring Anti Back-Out (ABO) feature provides increased resistance to screw back-out without compromising the biomechanical principles of the Integrated Interbody construct.
- Texturized surface topography provides 20x the roughness of machine finished PEEK providing enhanced stability upon implantation.
- 3-dimensional surface profile designed to provide enhanced cellular attachment and proliferation vs rougher titanium surfaces, with 2x the cellular attachment of courser titanium surfaces and 20x the cellular proliferation of other titanium surfaces.
- Available in multiple footprint and height configurations.
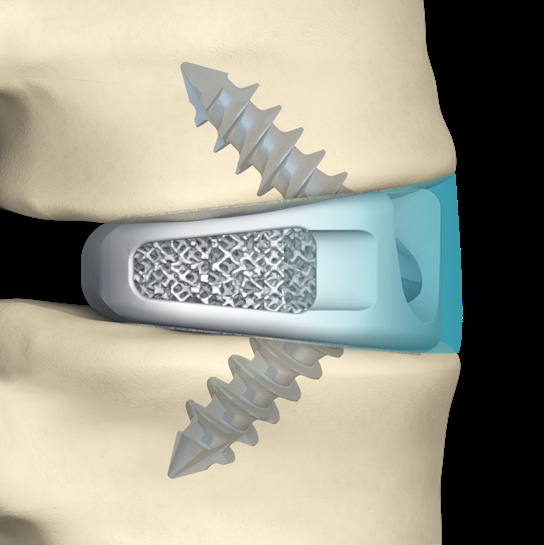
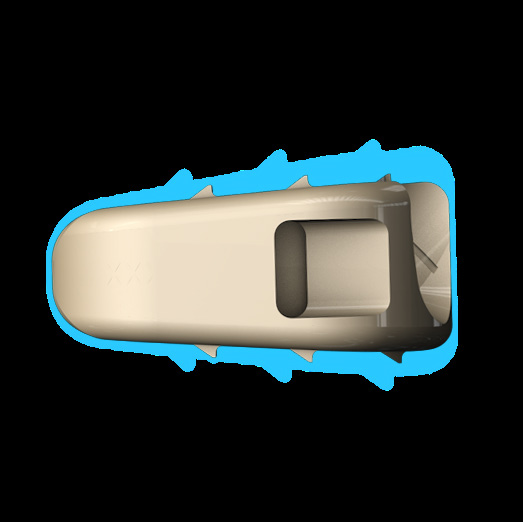
- 'No Profile' design allows the deviceto seat fully within the confines of the vertebral body.
- Leaves the anatomy unchanged external to the interbody
- Anatomical shape that is designed to sit on the apophyseal ring and better fill the disc space.
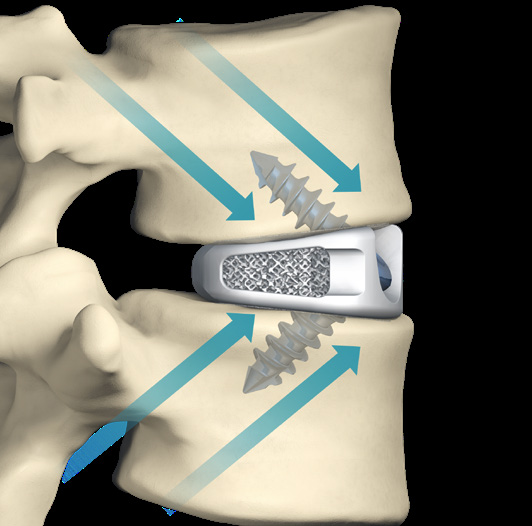
- Cancellous screws produce a lag effect between the vertebral body and the implanted device.
- Following Wolff's Law, the lag effect provides constant compressive forces against the implant and graft material to promote fusion
- The large graft area, combined with an anatomical footprint and load-sharing design, affords the bestopportunity for fusion.
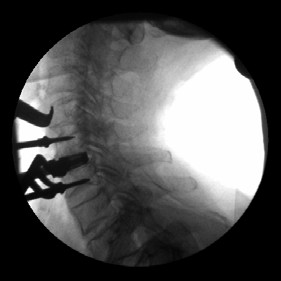
- Implants afford accurate postoperative assessment of fusion development.
- The Ti-ACTIVE surface allows for radiographic visualization of the graft-endplate contact.
- The STALIF C and STALIF C-Ti devices are intended to be used as an intervertebral body fusion cage as a standalone system used with bone screws provided and requires no additional supplementary fixation systems.
- The device system is designed for use with autograft bone and/or allogenic bone graft composed of cancellous and/ or corticocancellous bone graft to facilitate fusion.
- STALIF C and STALIF C-Ti are intended to be used at one or two contiguous levels.
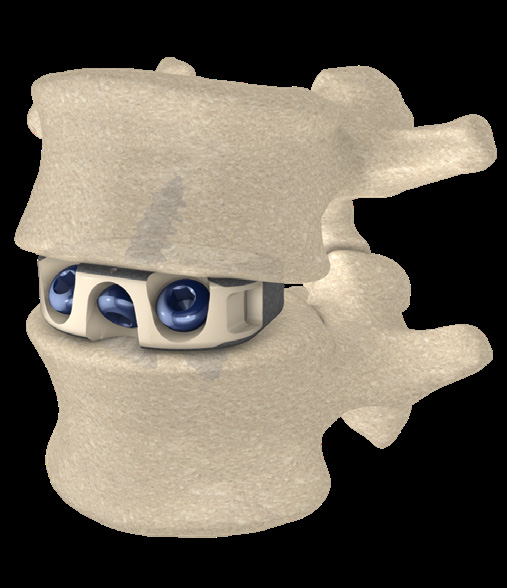
STALIF C TM is Stand Alone Cervical IBF Device. STALIF C™ is intended to be used as an IBF cage without supplementary fixation.
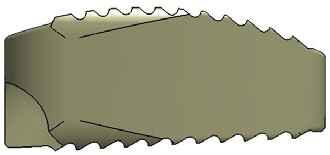
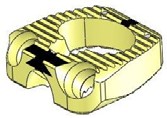

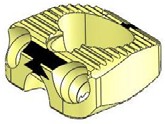
- Provides immediate mechanical stability with resistance to axial, torsional and bending movements
- Similar rigidity to a cage with anterior cervical plate or allograft with anterior cervical plate
- 3 screws
- Teeth for rotational stability
- Device implanted fully within confines of vertebral body
- No soft tissue irritation
- Large bone graft surface area to promote superior fusion
- Device sits on cortical shell
- Manufactured from radiolucent PEEK-Optima®
- Young's modulus similar to cortical bone
- Load sharing to promote fusion in line with Wolff's law
- Titanium markers for positioning
- MRI compatible
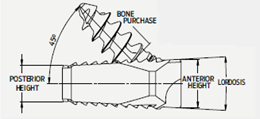
- Multiple heights available, 5.5mm - 9.5mm in 1.0mm increments
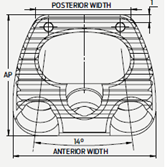
- AP length 14mm
- Medio-lateral dimension of 16.5mm
- 6° inclusive angle
- 2 sagittal profile options, Domed and Tapered
- Oblique skin crease incision, or
- Vertical incision over the medial border of the sternocleidomastoid
- Parallel core revision screws
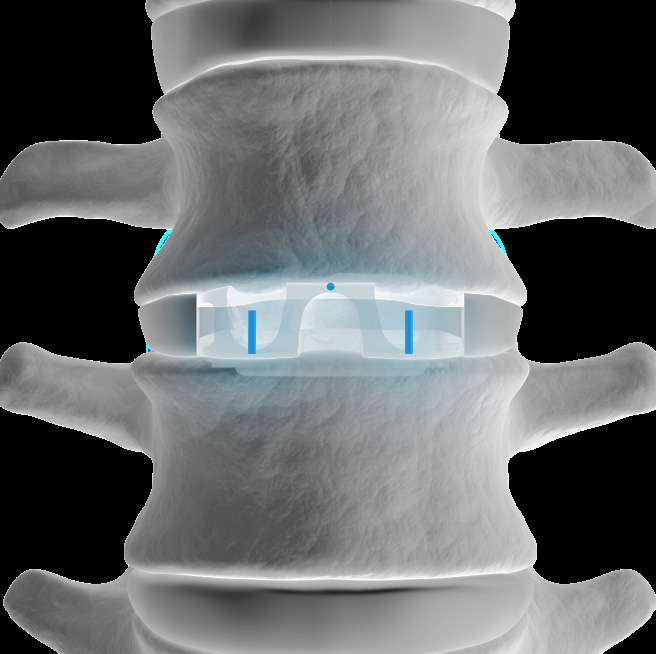
The STALIF M Portfolio is a comprehensive set of instruments and implants designed to support an anterior approach to the lumbar spine. The STALIF M portfolio No-Profile® implant designs are engineered to conform to patient anatomy, simplify surgery, enhance stability, and maximize opportunities for fusion. STALIF M portfolio Integrated Interbody ™ lumbar fusion devices are designed to achieve immediate load-sharing and segmental stability. Innovative implant design complemented by unique cancellous screws co-function during the healing process to form an “integrated” fusion construct that is highly stable. Laboratory evaluations have repeatedly shown that the STALIF M product family attains equivalent biomechanical performance to anterior plate and cage technologies, as well as anterior cage and posterior pedicle screw constructs. STALIF M portfolio implants are provided in 2 state of the art material options, including Ti-ACTIVE™ (microporous texturized titanium surface), and PEEK (poly-ether-ether-ketone). Unlike other standalone implants, STALIF ® devices are manufactured according to a specific biomechanical design rationale. This rationale was established with the original STALIF device introduced over 30 years ago. Through its multiple product iterations, STALIF has remained consistent and steadfast with the original design rationale. STALIF M-Ti is an innovative, titanium-surfaced, PEEK Integrated Interbody device design that blends the benefits of titanium and PEEK integrated interbody devices. PEEK has superior visualization properties and a modulus of elasticity that is similar to that of cortical bone while titanium is cell-friendly and enables human mesenchymal stem cells to adhere to the surface and proliferate. The Ti-ACTIVE surface topography is designed to provide enhanced cellular attachment and proliferation vs rougher titanium surfaces, enhancing opportunities for fusion.

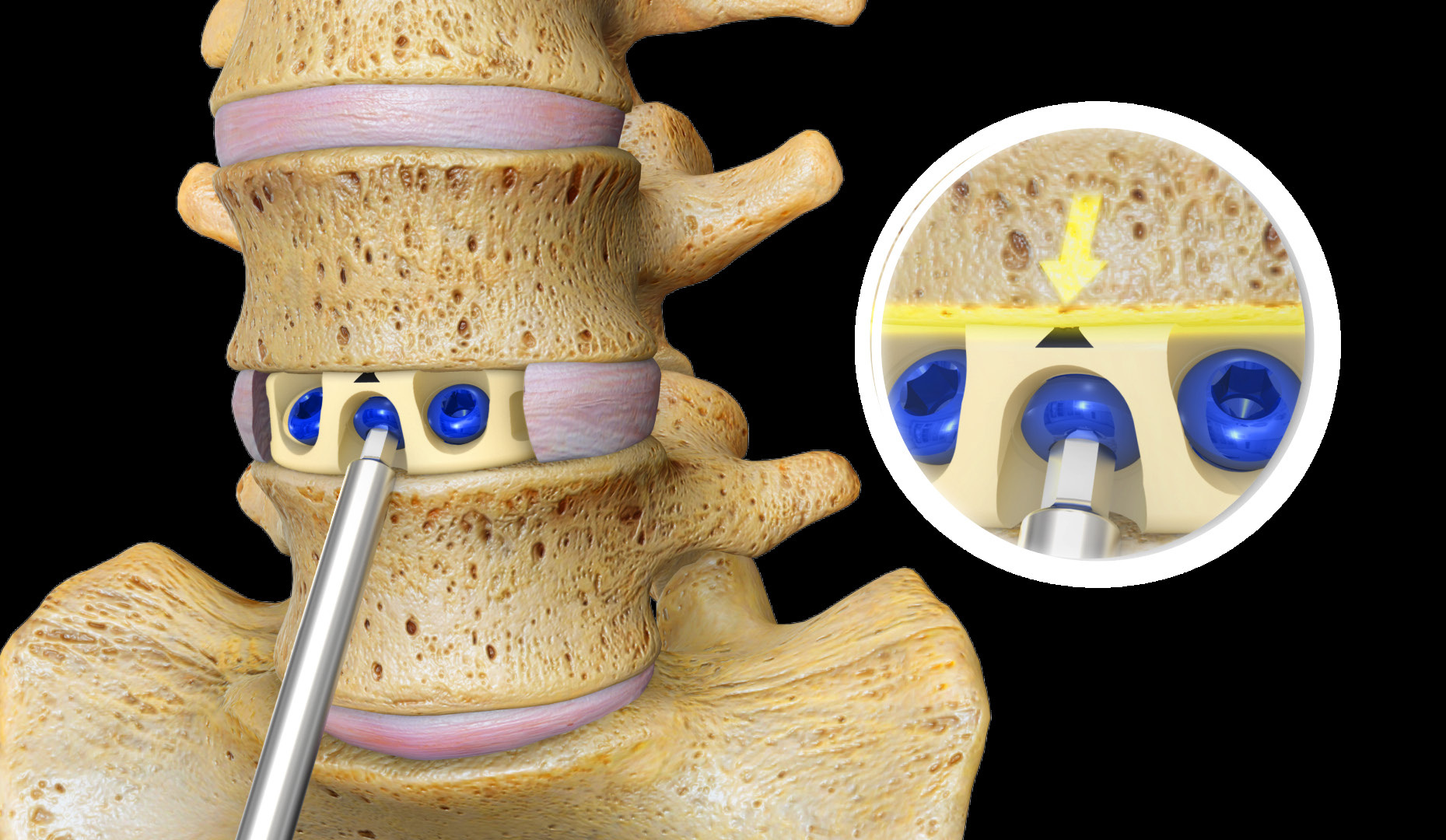
Stalif M TM and Stalif L
During anterior or lateral spinal fusion surgery, the painful spinal disc is reached with caution. The spine may then be stabilized using a spacer implant that is covered by a metal plate that bridges the vertebral bodies. Alternatively, the surgeon may utilize our more advanced technology—where a single device, STALIF M or STALIF L, replaces the disc portion of the damaged spinal segment. The STALIFdevice is then secured with specially-designed screws. The procedure encourages fusion between the vertebrae above and below the affected spinal segment. This type of surgery generally requires less operative time, and more importantly, leaves all of the bones and muscles of the back intact. This may result in a shorter recovery time, allowing the patien to return to his daily activity more quickly. STALIF M and STALIF L are available in multiple material options to meet each individual patient's needs. Available devices include:
STALIF M
- Anterior fusion device for the lower (lumbar) spine, available in 3 material options(PEEK, PEEK with Ti-ACTIVE™, and FLX™).
STALIF L
- Lateral fusion device for the lower (lumbar) spine (L2-L5), available in 2 material options (PEEK and FLX).
PEEK
- PEEK (polyetheretherkeytone) is a high performance medical grade plastic.
FLX
- FLX is a 3D-printed porous titanium alloy scaffold.
Ti Active
- Ti-ACTIVE is a three-dimensional microporous commercially-pure titanium surface.
- PEEK and Ti-ACTIVE implants also include Tantalum markers. All STALIF integrated fixation screws are made of titanium alloy. All materials used in the production of STALIF devices are highly biocompatible. Please consult with your doctor regarding any possible allergies.
- Titanium has been shown to improve the characteristics of orthopedic and spinal implants.
