Medacta
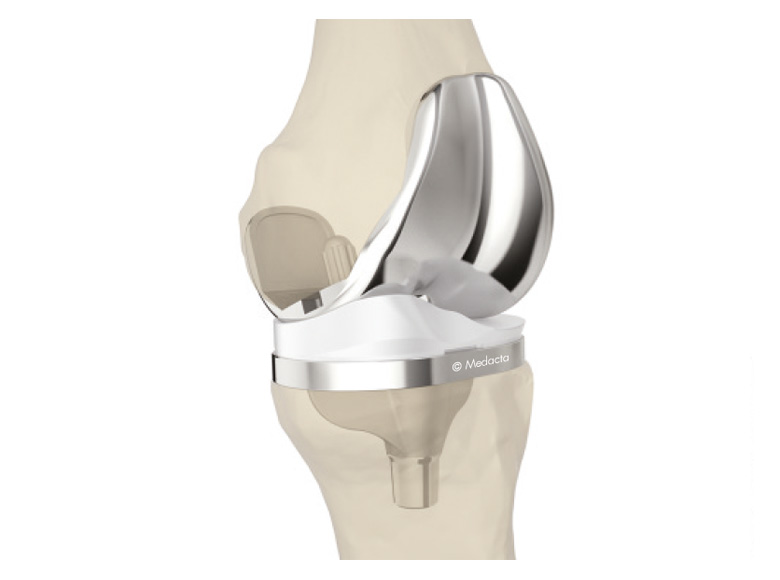
GMK Primary
GMK SYSTEM has been conceived to provide surgeons with a complete range of knee arthroplasty options, aiming to fulfill pecific patient needs. The strength of the system is the synergy between the available solutions: The femoral components of primary and revision prosthesis have the same internal profile, ensuring maximum intra-operative flexibility to address different surgical scenarios. The modularity of the GMK instruments allows the surgeon to better adapt his/her approach to specific situations.






- Complete product range: cruciate retaining, ultracongruent and posterior-stabilised.
- Both mobile and fixed bearing version available.
- Bone preserving design that avoids the femoral box.
- Anatomic deep design of the trochlea groove.
- The GMK Primary is designed for cementless and cemented use in total knee arthroplasty, if there is evidence of sufficient sound bone to seat and support the components.
- This knee replacement system is indicated in the following cases:
- Severely painful and/or disabled joint as a result of arthritis, traumatic arthritis, rheumatoid arthritis or polyarthritis.
- Avascular necrosis of femoral condyle.
- Post traumatic loss of joint configuration.
- Primary implantation failure.
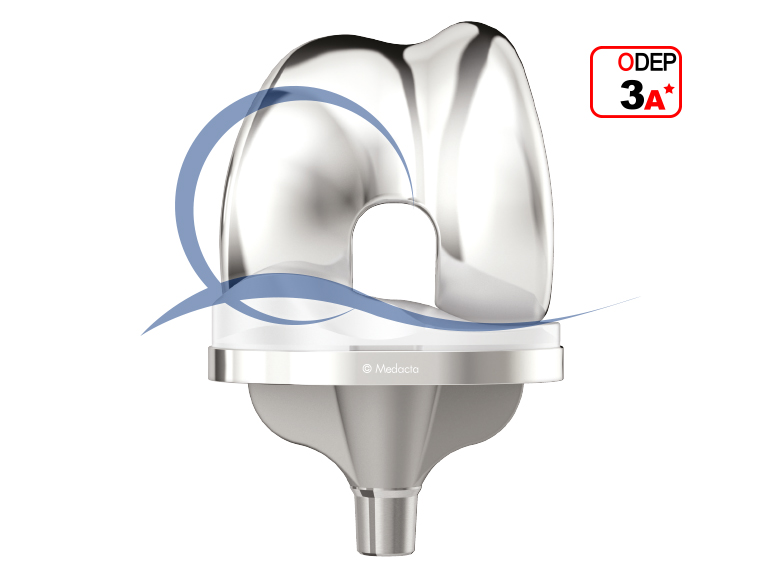
GMK Sphere
Based on the knee anatomy and kinematic studies conducted by Prof. Michael Freeman and Prof. Vera Pinskerova, GMK Sphere is an innovative total knee implant designed to deliver maximum functional stability with the goal of increasing TKA patient satisfaction during activities of daily living and decreasing post operative knee pain.

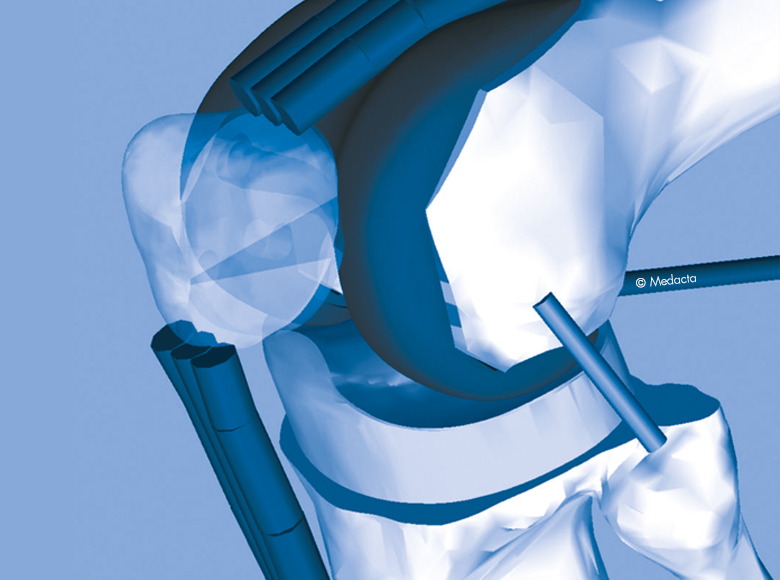


Stability
- GMK Sphere features a fully congruent medial compartment providing High stability throughout the range of motion
Natural Patellar Tracking
- GMK Sphere replicates the natural lateralized patella tracking to reduce patellofemoral joint pressure and address anterior knee pain
Anatomical Fit
- The combination of 13 femoral sizes and inserts with 1 mm increments allows the surgeon to “fine tune” ligament balance and improve stability throughout the range of motion.
- The GMK Sphere is designed for cementless and cemented use in total knee arthroplasty, if there is evidence of sufficient sound bone to seat and support the components.
This knee replacement system is indicated in the following cases:
- Severely painful and/or disabled joint as a result of arthritis, traumatic arthritis, rheumatoid arthritis or polyarthritis.
- Avascular necrosis of femoral condyle.
- Post traumatic loss of joint configuration.
- Primary implantation failure.
Revision
The GMK Revision System has been designed with a clear goal: minimize complexity, maximize versatility. Same internal femoral profile across all GMK system implants. allows for a full transition through the system, providing incremental constraint according to each patient's need.


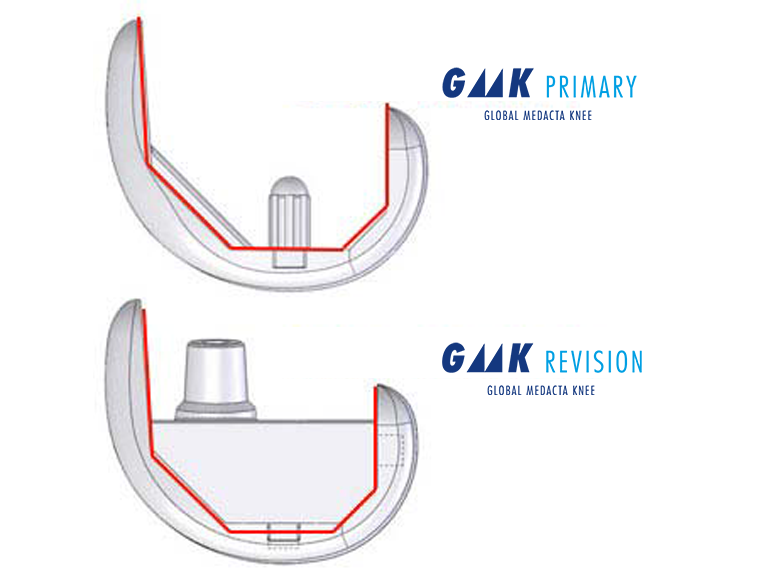
various levels of constraint available
- The same femoral articular profile allows for full compatibility with GMK Primary inserts thus providing various levels of incremental constraint: ultra-congruent, posterior stabilized and semi-constrained.
Bone Preserving
- Requiring minimal condylar resections and a reduced intercondylar box.
360° OFFSET
- On the tibial side, in combination with the asymmetric tibial baseplate, the offset option helps obtain uncompromised coverage of the tibial plateau profile.
- On the femoral side, the offset option helps optimise the position of the implant relative to the intramedullary canal to accurately restore anterior flange location and flexion gap balance.
Comprehensive Range Of Sizes And Options
- Cemented and cementless extension stems, interchangeable between tibia and femur, are available to address different patient needs and surgeon preferences.
- Augmentation blocks, interchangeable between medial and lateral side, are available both for tibia and femur to address asymmetrical bone defects.
- Various thicknesses are available for tibial inserts and tibial femoral augments to restore the appropriate joint line
- The GMK Revision knee prosthesis is designed for cemented use in total knee arthroplasty
Goals of a successful total knee revision include, amongst others:
- mechanical alignment restoration
- re-establishment of the joint line
- good fixation of revision implant components
- restoration of an acceptable range of motion
- flexion/extension gap balancing
Amis
The AMIS STEM has been developed to facilitate broaching and stem insertion when utilizing the AMIS approach without compromising implant stability, Based on the Clinical experience of Straight, rectangular, cementless hip stem hip stems, The AMIS Stem Incorporates features which simplify the AMIS approach.

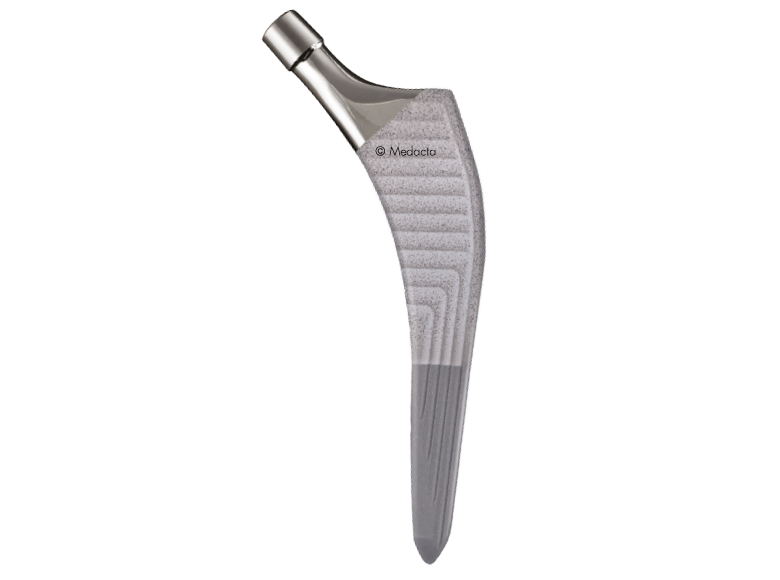

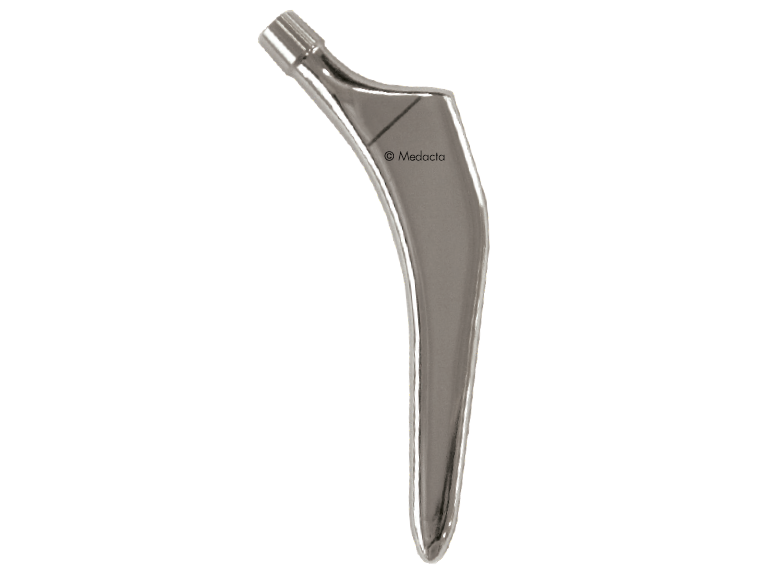


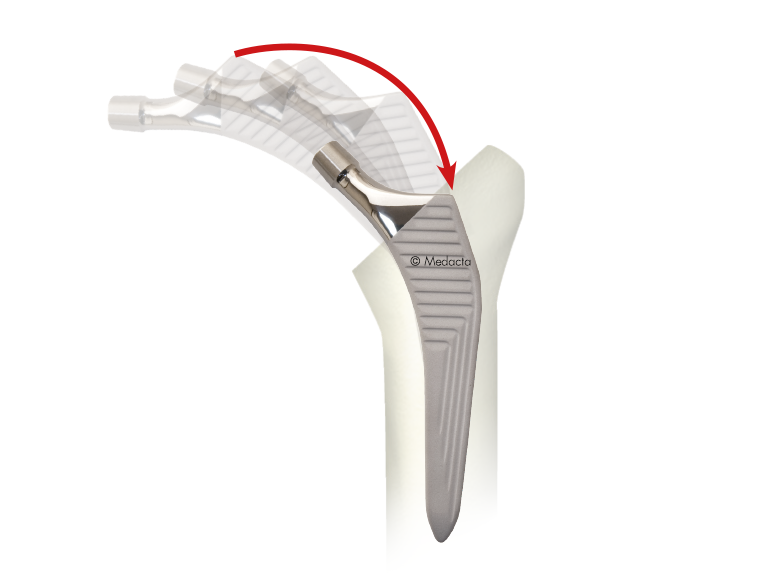
- First Stem Specifically designed for AMIS
- Easier stem introduction due to reduced lateral flare
- Overall dimensions reduced by 33% compared to standard straight rectangular stems
- Proven Stability for both cementless and cemented versions in biomechanical tests
- Reduced bone removal due to optimized length
AMIStem hip prostheses are designed to be used in total or partial hip arthroplasty, for primary or revision surgery. Total hip arthroplasty is indicated in the following cases:
- Severely painful and/or disabled joint as a result of arthrosis, traumatic arthritis, rheumatoid polyarthritis or congenital hip dysplasia
- avascular necrosis of the femoral head
- acute traumatic fracture of the femoral head or neck
- failure of previous hip surgery: joint reconstruction, internal fixation, arthrodesis, partial hip arthroplasty, hip resurfacing replacement or total hip arthroplasty
Apricot
With a clinical history starting in 2003 and thousands of stems implanted every year worldwide, Quadra System stems have proved to be a reliable solution for hip arthroplasty. In a clinical study by Balgrist University, a 100% survival rate at 5 years was reported on 109 patients, considering aseptic loosening as the endpoint.

- Complete range of straight stems
- Effective stability thanks to the triple tapered design
- Wide range of sizes
- Close contact between the stem and the cortical bone thanks to the tapered shape and high precision broaches.
- Good stability.
- Natural load transfer.
- Minimised stress-shielding risk, preserving healthy bone.
- Non-bulky lateral shoulder ideal for MIS approaches.
- Reliable, compact and precise instrumentation
The Quadra System is designed to be used in total or partial hip arthroplasty, for primary or revision surgery Total hip arthroplasty is suitable in the following cases:
- Severely painful and/or disabled joint as a result of arthrosis, traumatic arthritis, rheumatoid polyarthritis or congenital hip dysplasia
- Avascular necrosis of the femoral head
- Acute traumatic fracture of the femoral head or neck
- failure of previous hip surgery: joint reconstruction, internal fixation, arthrodesis, partial hip arthroplasty, hip resurfacing replacement or total hip arthroplasty
partial hip arthroplasty is suitable in the following cases:
- Acute traumatic fracture of the femoral head or neck
- Non-union of femoral neck fracture
- Avascular necrosis of the femoral head
- primary pathology involving the femoral head but with a non deformed acetabulum
Femoral Head
Small grain size and high uniformity make MectaCer BIOLOX delta heads very hard and smooth leading to increased wettability and reduced wear. The complex grain structure helps to reduce the risk of propagation of cracks and increases overall strength. The titanium insert on the Option revision head provides a pristine taper for revision cases.




- The microstructure of MectaCer BIOLOX delta ceramic has three different size grains: alumina, zirconia and strontium.
- The zirconium oxide particlesact like “airbags” by absorbing impacting forces and the platelet shaped crystals help to reduce the propagation of cracks.
Mpact
The Mpact System is a modular cementless hemispherical acetabular shell providing the choice between different shell sizes, liner shapes and materials. Mpact offers a system of hemispherical press-fit acetabular shells in titanium alloy that deliver different solutions according to patient needs, addressing primary and revision indications. Cementless hemispherical shell design with porous coating surface treatment has a long and successful clinical history. The Mpact shells follow this philosophy, enhancing primary stability and biological secondary fixation with the Mectagrip, highly porous plasma spray coating.Mpact's hemispherical geometry and its firm press-fit provide an excellent primary stability which Could be enhanced, if necessary, by adding screws (Mpact Two-hole and Multi-hole).

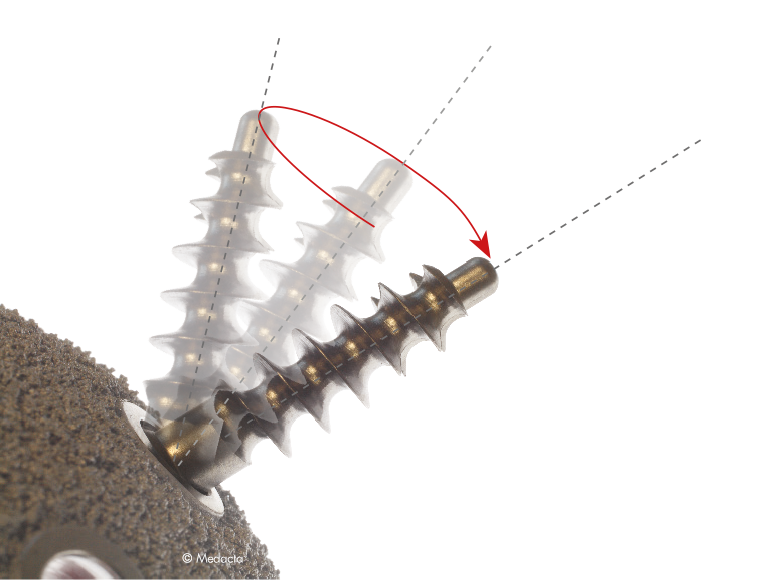








- Optimal Primary Stability and Secondary Fixation
- Locking System for The Liner which minimizes micro-movements, preventing backside wear
- Ceramic Liners for Revision Cases
- Easy to use Instrumentation
- Multiple Shell Versions: Available to secure adequate fixation to the available bone stock
- Optimized Femoral Head/Shell Diameter Ratio
The Mpact acetabular shell is designed to be used in total hip arthroplasty, for primary or revision surgery:
- Severely painful and/or disabled joint as a result of arthrosis, traumatic arthritis, rheumatoid polyarthritis, or congenital hip dysplasia.
- Avascular necrosis of the femoral head.
- Acute traumatic fracture of the femoral head or neck.
- Failure of previous hip surgery: joint reconstruction, internal fixation, arthrodesis, partial hip arthroplasty, hip resurfacing replacement, or total hip arthroplasty
Quadra
With a clinical history starting in 2003 and thousands of stems implanted every year worldwide, Quadra System stems have proved to be a reliable solution for hip arthroplasty. In a clinical study by Balgrist University, a 100% survival rate at 5 years was reported on 109 patients, considering aseptic loosening as the endpoint.
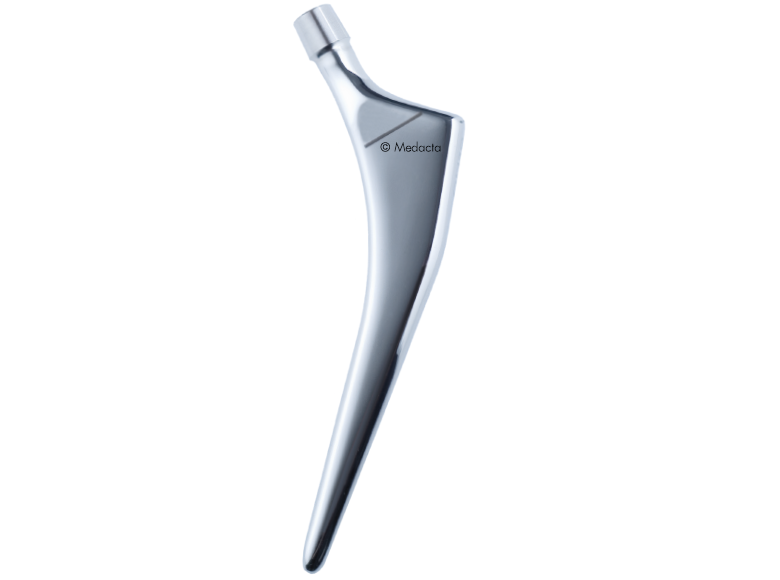




- Complete range of straight stems
- Effective stability thanks to the triple tapered design
- Wide range of sizes
- Close contact between the stem and the cortical bone thanks to the tapered shape and high precision broaches.
- Good stability.
- Natural load transfer.
- Minimised stress-shielding risk, preserving healthy bone.
- Non-bulky lateral shoulder ideal for MIS approaches.
- Reliable, compact and precise instrumentation
The Quadra System is designed to be used in total or partial hip arthroplasty, for primary or revision surgery Total hip arthroplasty is suitable in the following cases:
- Severely painful and/or disabled joint as a result of arthrosis, traumatic arthritis, rheumatoid polyarthritis or congenital hip dysplasia
- Avascular necrosis of the femoral head
- Acute traumatic fracture of the femoral head or neck
- failure of previous hip surgery: joint reconstruction, internal fixation, arthrodesis, partial hip arthroplasty, hip resurfacing replacement or total hip arthroplasty
partial hip arthroplasty is suitable in the following cases:
- Acute traumatic fracture of the femoral head or neck
- Non-union of femoral neck fracture
- Avascular necrosis of the femoral head
- primary pathology involving the femoral head but with a non deformed acetabulum
MyKnee is a set of 3D printed patient-specific guides that allow accurate and reproducible implant placement based on a pre-operative 3D plan, that has been created from the CT or MRI images of the patient's knee. This innovative concept combines multiple features that support benefits for both the surgeon and the patient.
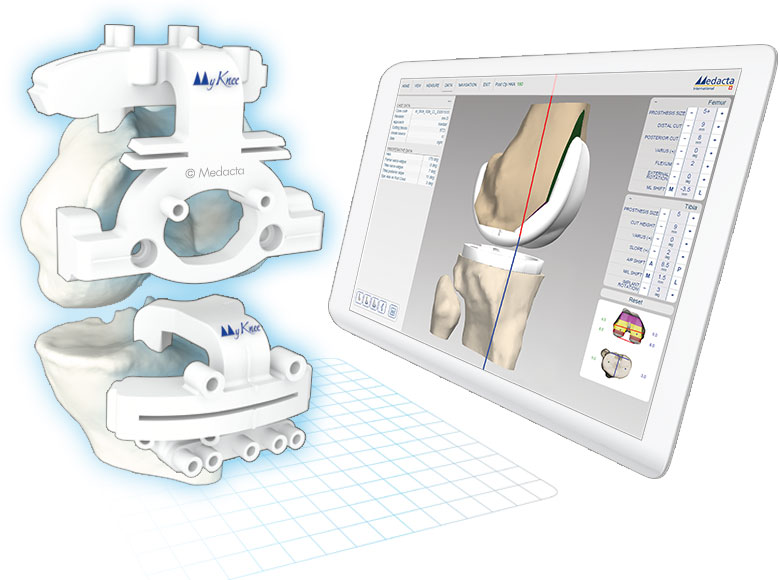
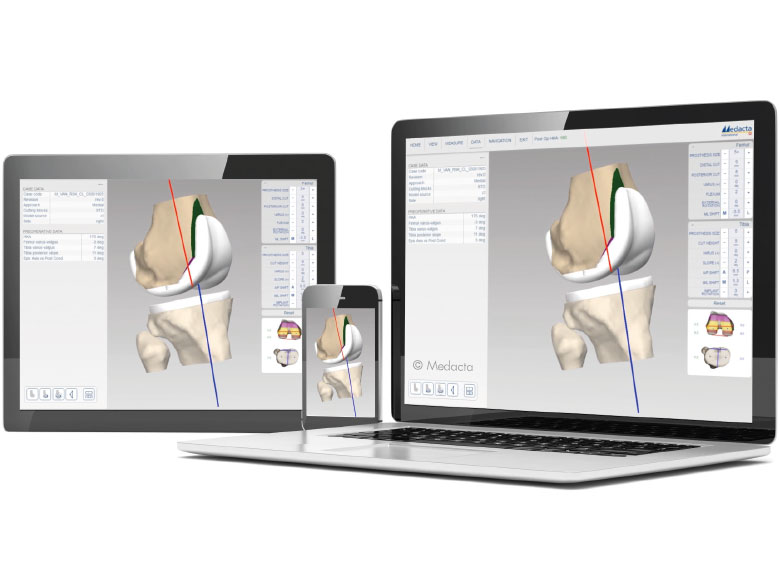
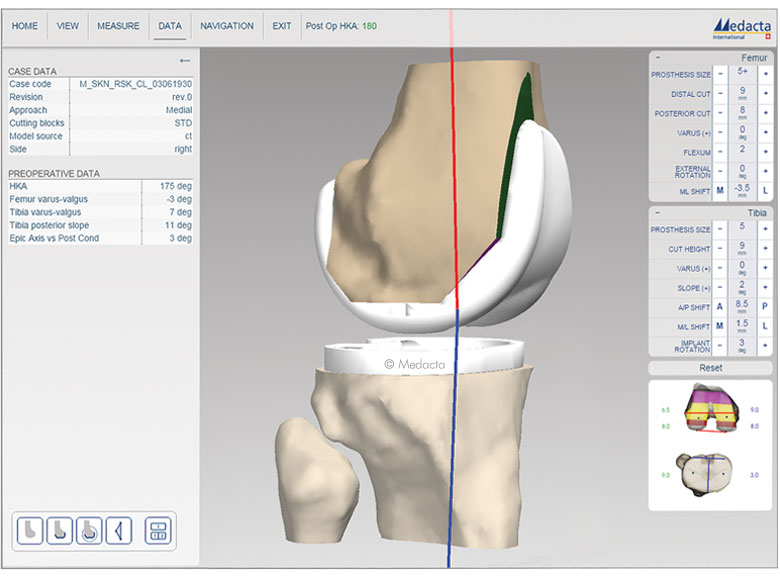
- Accurate implant positioning
- No intramedullary canal violation with less bleeding and haemoglobin
- Up to 60% reduction of surgical steps and related time for bone resection
- Potentially one extra case per surgery session Proven Stability for both cementless and cemented versions in biomechanical tests
- Comfort of use in every surgical scenario, also in total knee revision cases
- Interactive 3D web planner
- With each case, the surgeon can modify femoral and tibial parameters, such as:
- Femoral distal, anterior-posterior resection levels, femoral rotation, femoral flexion and femoral varus/valgus.
- Tibial resection level and tibial varus/valgus.
- Goals of a successful total knee revision include, among others:
- Mechanical alignment restoration
- Re-establishment of the joint line
- Good fixation of revision implant components
- Restoration of an acceptable range of motion
- Flexion/extension gap balancing
The spine is one of the most important structures in the human body. It supports much of the body weight, provides points of attachment for muscles and ligaments, and protects the spinal cord, which carries information from the brain to the rest of the body. MySpine is a tailor made patient specific spine vertebrae guide, created to lead the surgeon through the critical steps of accurate pedicle screw placement whilst reducing the surgical time and intra-operative X-ray radiation. MySpine is intended as a thoracic and lumbar posterior pedicle targeting guide for patients requiring spinal fusion
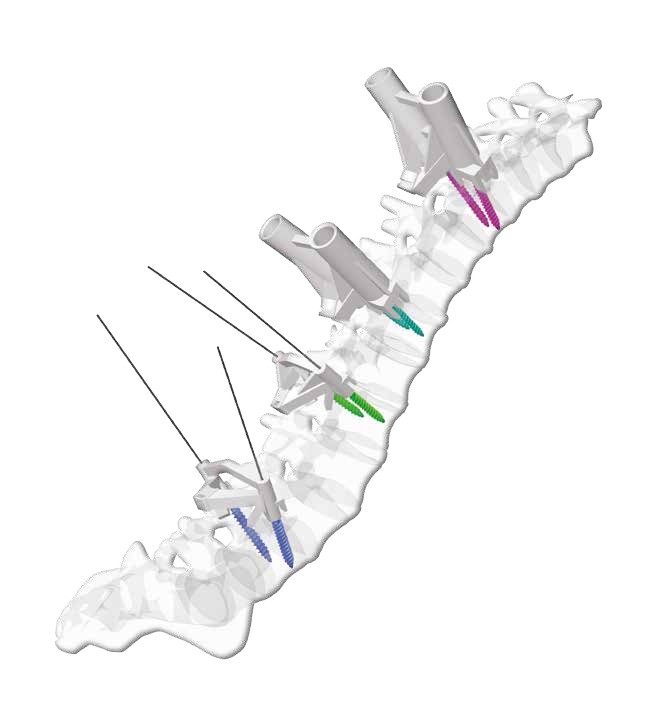
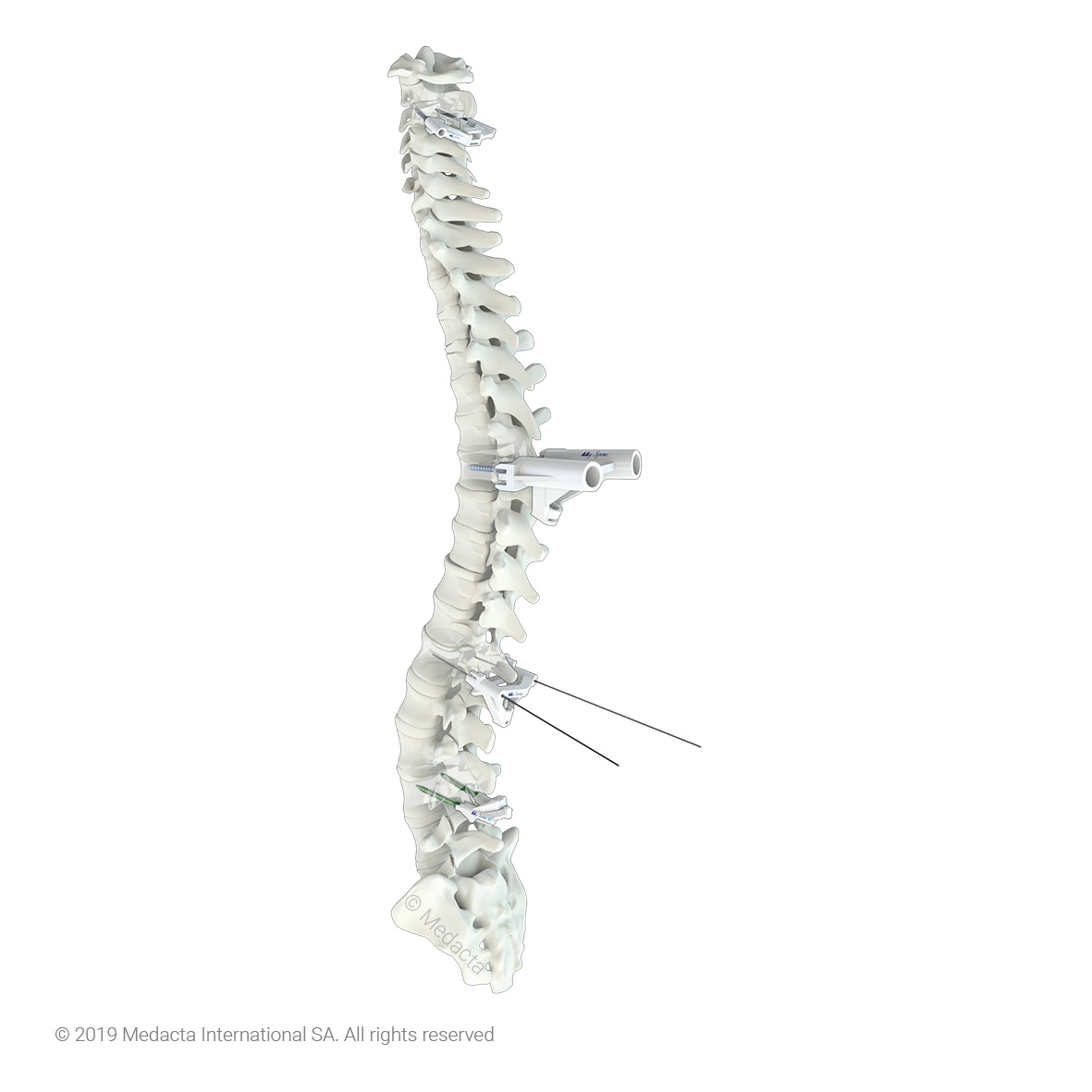
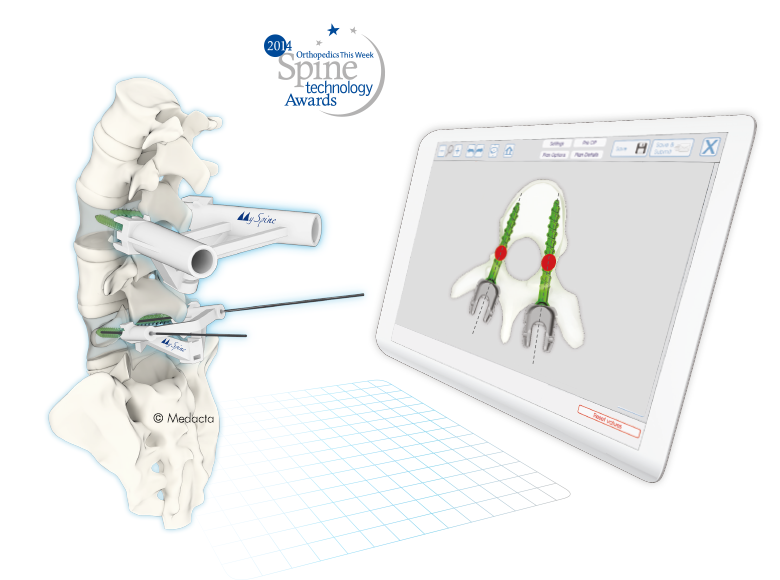
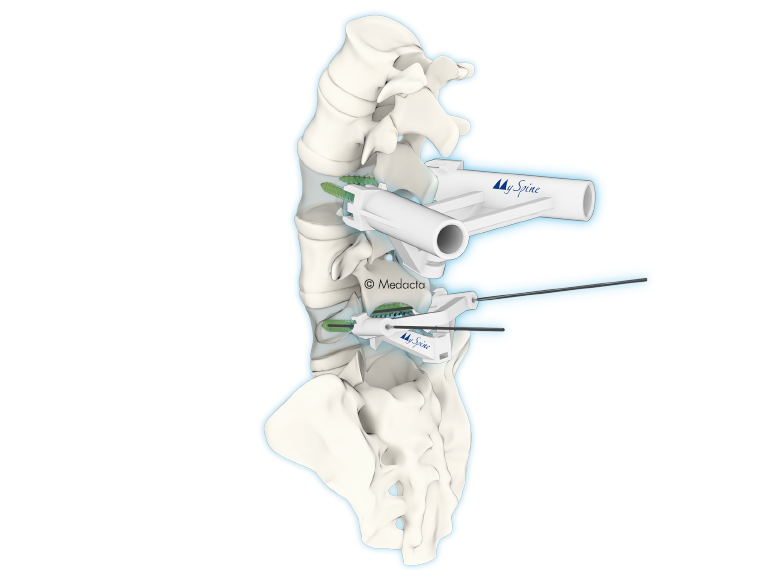
- MySpine guides are positioned on the vertebra usingdistinct references, such as the spinous and transverseprocesses, in order to achieve the maximum stability.
- The MySpine guides are designed to preserve thepatient's anatomy.
- Distal windows enhance the field of view during all surgical steps.
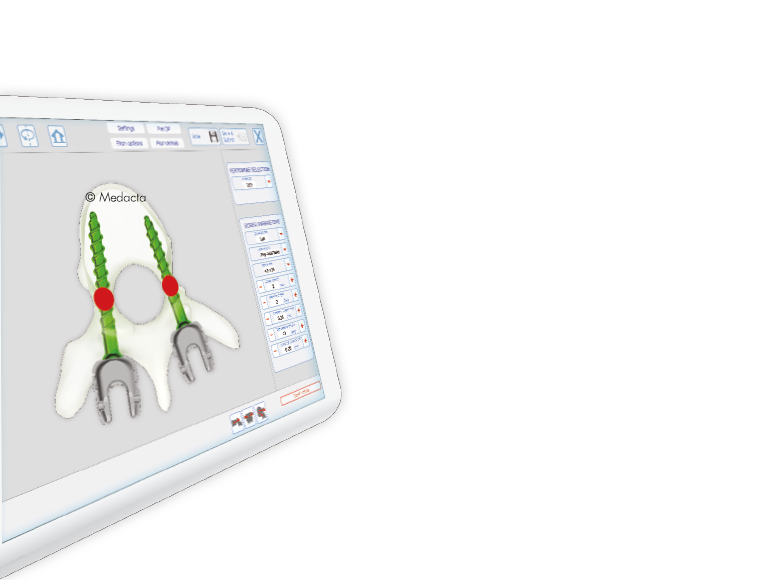
- MySpine is a patient matched, pedicle targeted technology involving the production of patient specific, guides for placement pedicle screws, based on the patient's anatomy.
- The MySpine platform allows the surgeon to complete preoperative planning in 3D based on the patient's spinal CT scans.
- Different screw placement guide configurations are available:
- Standard for pedicle screw guidance with conventional screw trajectory
- Low Profile: for K-wire guidance with conventional screw trajectory
- Drill Pilot: Low Profile for pedicle path preparation with conventional screw trajectory
- MC: Drill Pilot and K-wire guidance with cortical bone path
NextAR Spine assists the surgeon in precisely locating anatomical structures in either open / mini-open or percutaneous spine procedures for the safe placement of implants, especially in anatomically critical areas. The NextAR Spine platform tracks the patient's anatomy, continuously updating its position on patient-specific 3D Xray images, such as 3D C-Arm or 3D CT scan. The software displays 3D scan images on-screen from a variety of perspectives (axial, sagittal, coronal). The indication range of the NextAR Spine platform spans from thoracic to lumbosacral to pelvis surgery. The NextAR software creates a translation map between the intraoperative images and the corresponding points on the patient anatomy. Whenever the user touches a point on the patient's bone using a special tracking instrument, the computer identifies the corresponding point on the intraoperative scan. The NextAR Spine platform is intended as an aid for precisely locating anatomical structures in either open/mini-open or percutaneous spine procedures. It is indicated for any medical condition in which the use of stereotactic surgery may be appropriate, and where reference to a rigid anatomical structure, such as vertebrae or pelvis, can be identified relative to images of the anatomy. This can include the following spinal implant procedures, such as Pedicle Screw Placement, Iliosacral Screw Placement, Interbody Device Placement

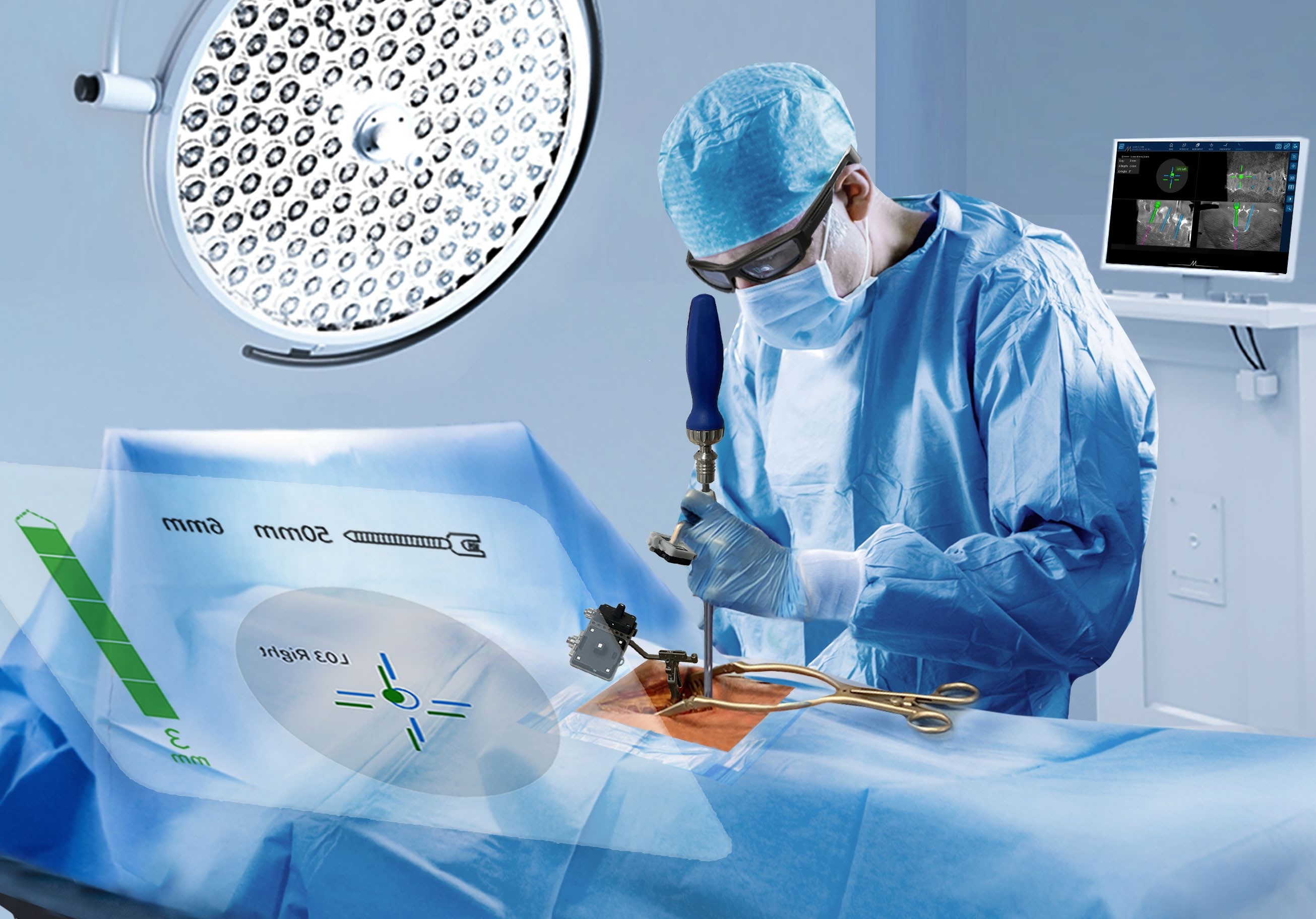
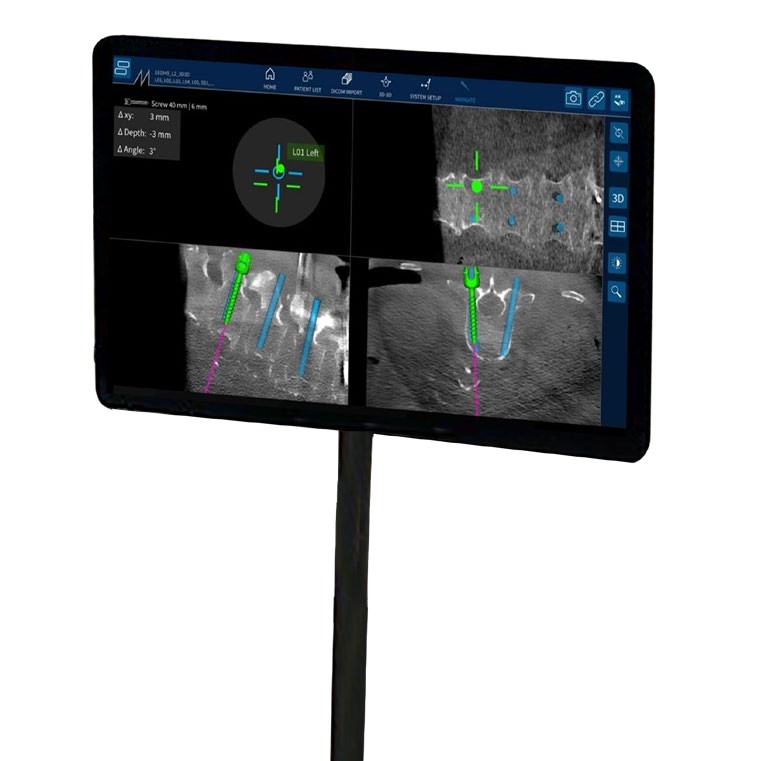
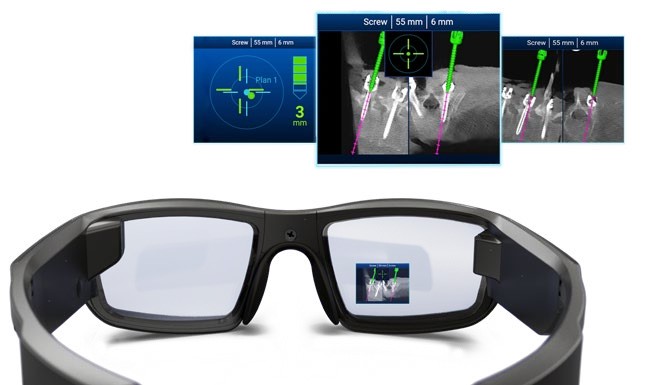

- Enhanced visualization to optimize surgery
- Data driven personalization
- Compact and integrated system
- Limit bleeding
- Reduce infection rates and postoperative pain
- Reduce the length of hospitalization
- Make rehabilitation easier and faster
- NextAR Spine addresses the need of a constantly increasing level of accuracy in Spine Surgery with a very lean and non-invasive workflow, maintaining a low capital investment and overall health care sustainability. This is accomplished by combining navigation with Augmented Reality and using as a tracking system smart tools that optimize the time of setup and the space needed in the Operating Room.
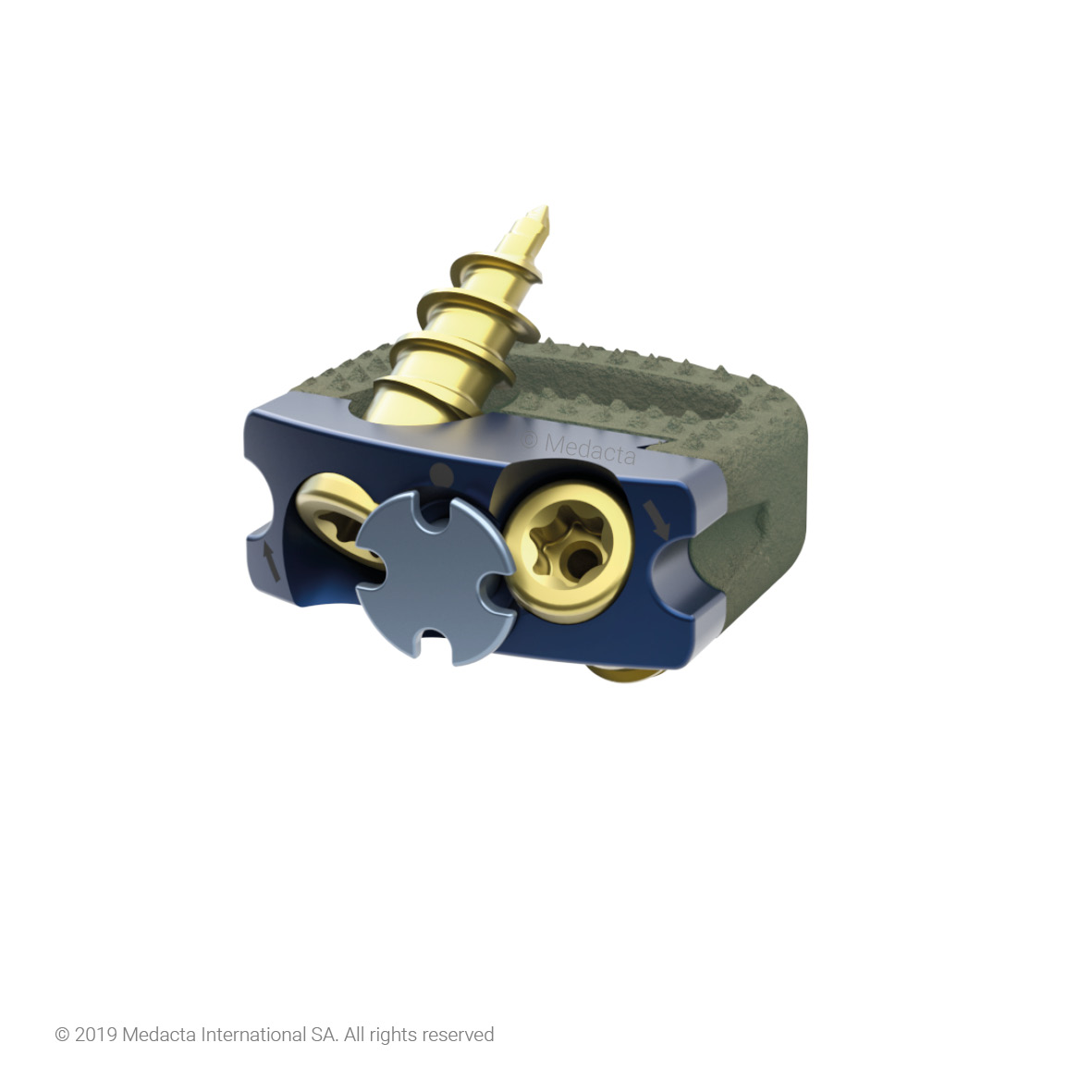
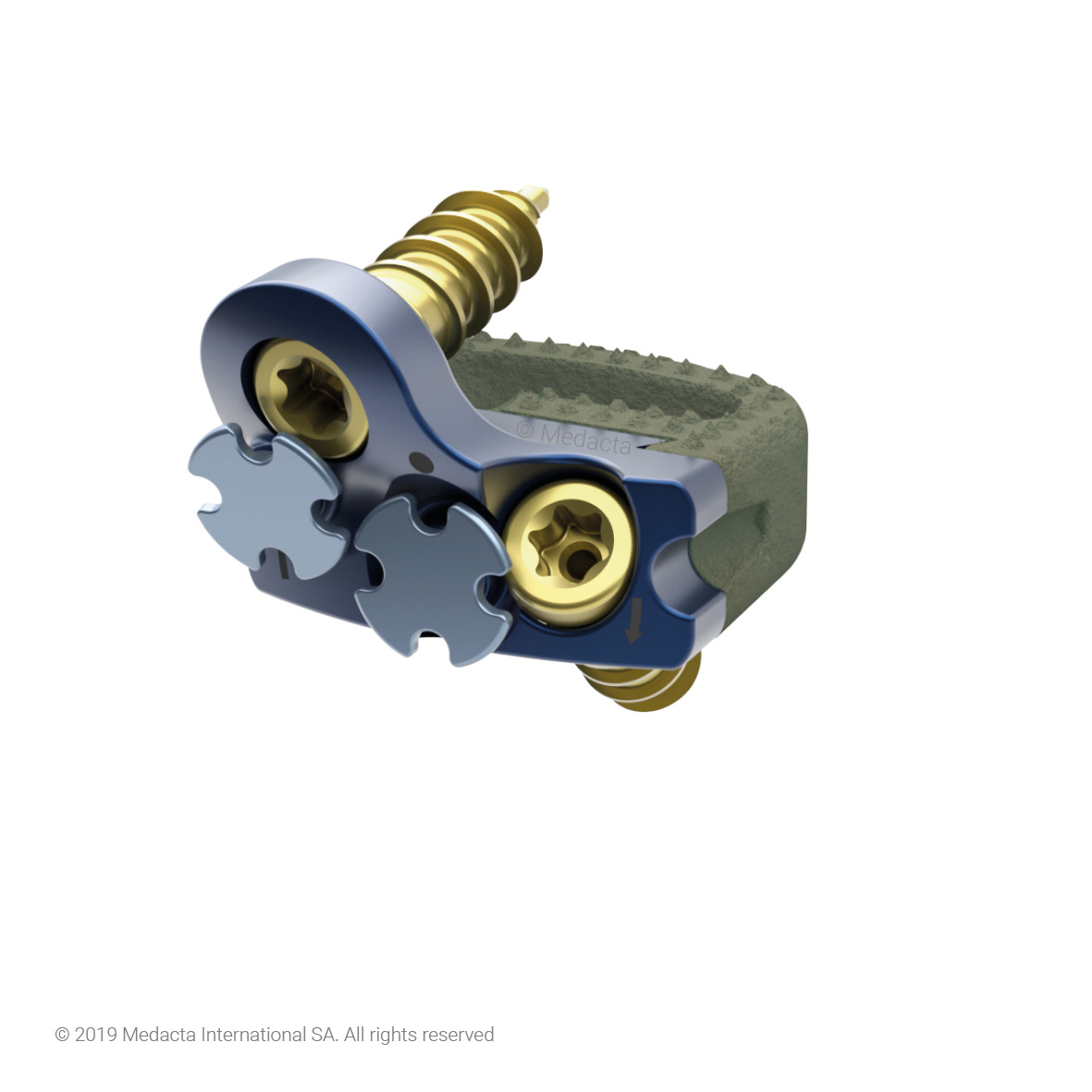
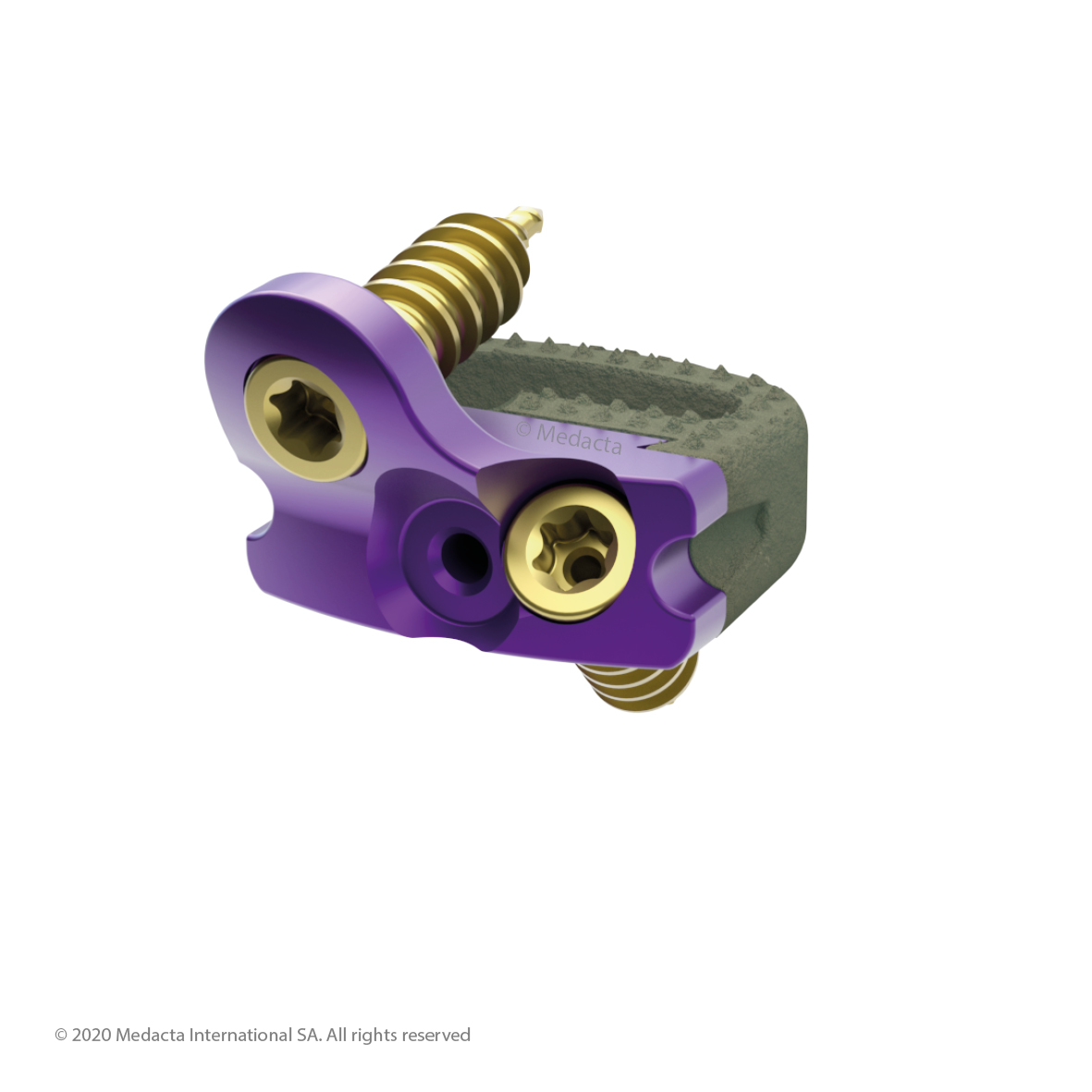
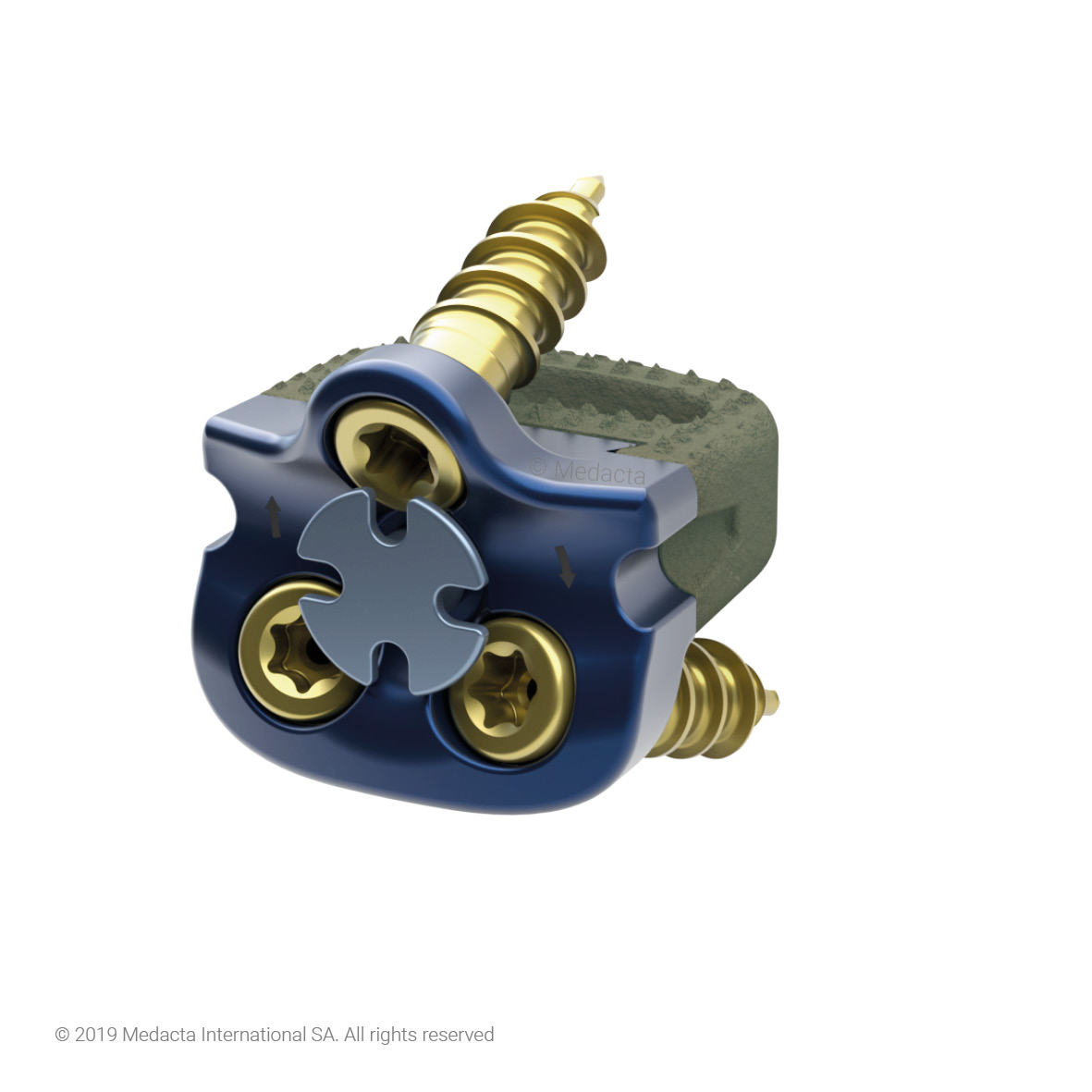
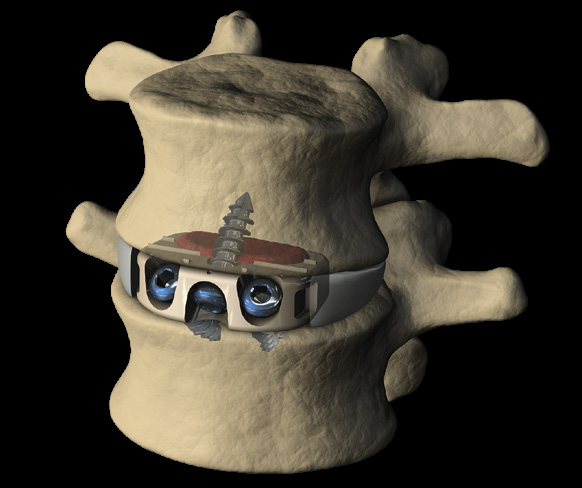
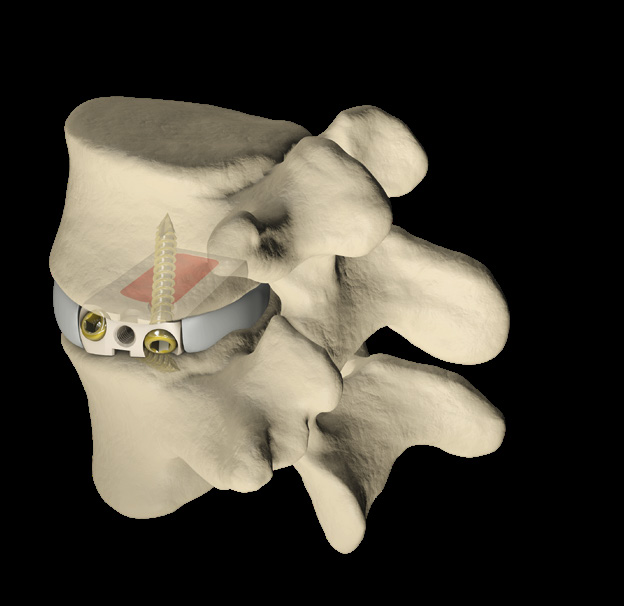
Mecta-C Stand Alone is a surgical solution specifically designed to provide multiple fixation options for patients undergoing anterior cervical discectomy and fusion surgery. Mecta-C Stand Alone System is specifically designed to provide your surgeon with multiple surgical options for correcting your spine. It offers 4 different fixation devices, in order to accommodate the patient-specific anatomical requirements. The Mecta-C Stand Alone system is a modular solution for Anterior Cervical Discectomy and Fusion (ACDF) that combines the benefits of an anterior plate and a radiolucent interbody spacer.



- Correct realignment of your spine and vertebrae
- Minimally invasive approach through a small incision
- Reduction in pain, recovery of mobility, and improvement in your quality of life
- Short hospital stay and fast recovery
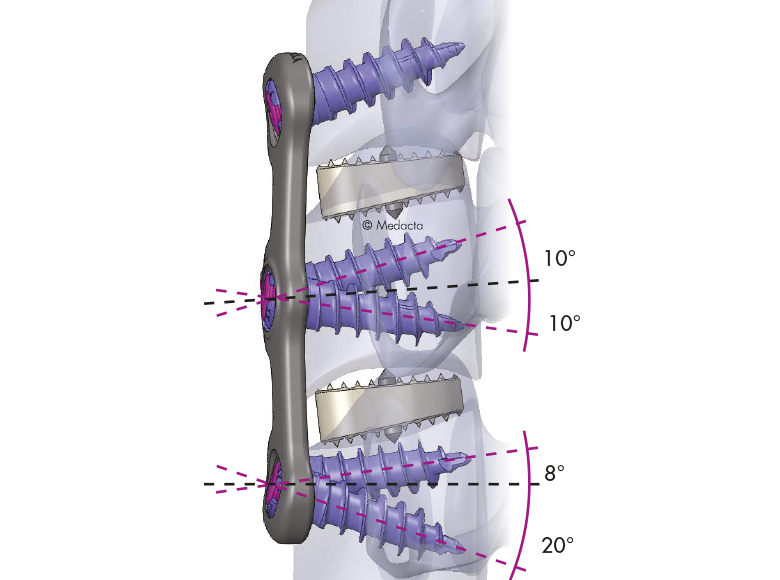
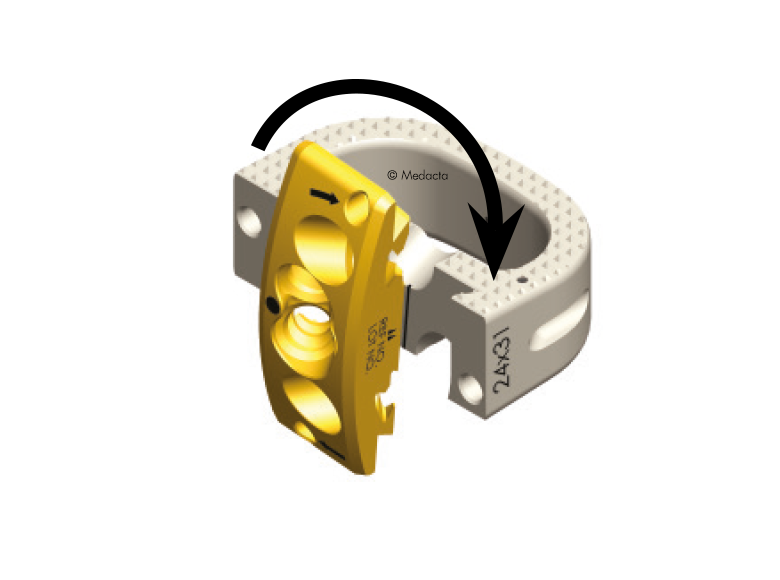
- The system is completely modular. It offers a cage with 3 different footprints and 8 heights. Lordosis is fixed to 7°. The surgeon can connect the selected cage with any of the 4 profile plates in order to accommodate specific anatomical requirements
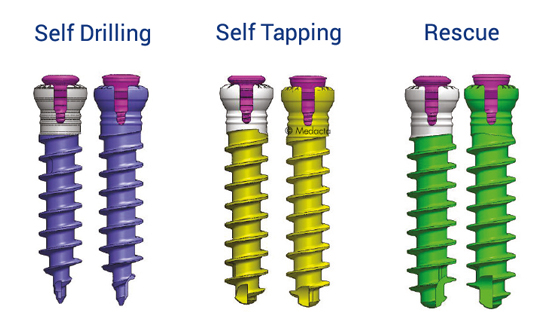
- Locking and lag screws are offered to further complete the system. For each design both self-tapping and self-drilling screws are available. Locking screws are compatible with rigid fixation plate while lag screws are compatible with variable fixation plate. Further fixation is required in the variable fixation construct with the antibackout screw.





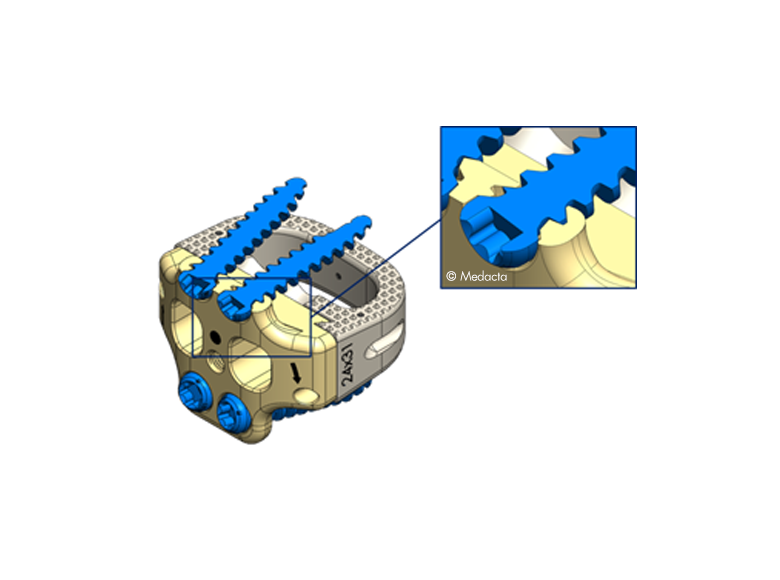
- The pyramidal teeth & lateral spikes allow:
- Grip the endplates
- Avoid migration
- Provide multi-directional
- expulsion resistance
- Two different anatomical designs to restore the disc space height and lordosis according to specific patient needs. The physiologic design and the range of available cage sizes allow the surgeon to choose a cage that matches the patient's unique individual anatomy.
- Large central bone graft area helps to accelerate the occurrence of fusion through the implant
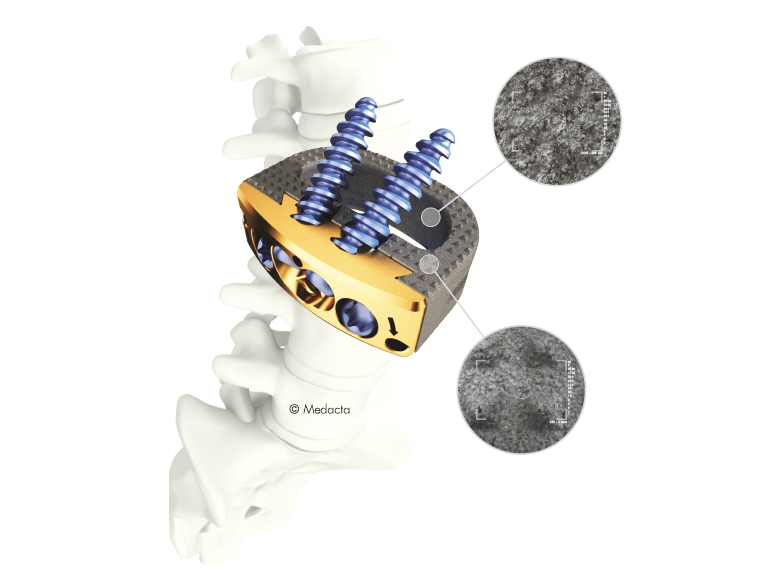
- The Mecta-C Cage System consists of PEEK and titanium coated PEEK intervertebral fusion devices capable to offer effective load sharing and optimal biocompatibility.
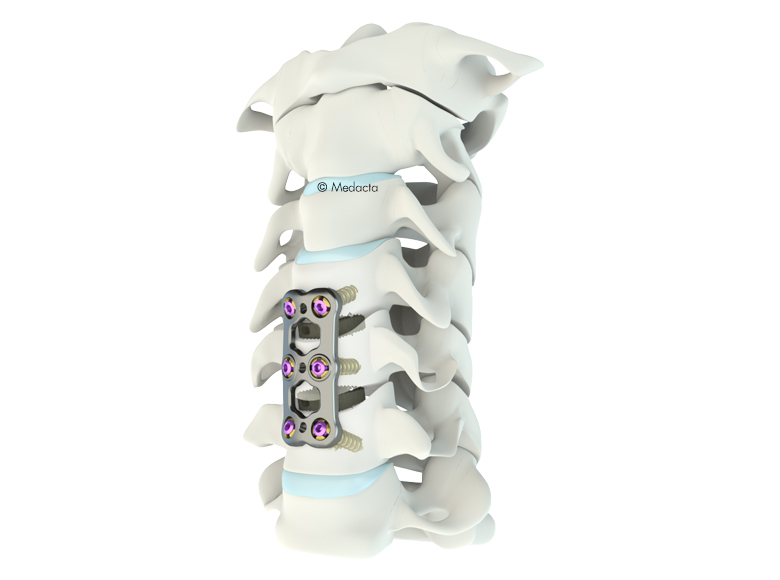
The Mecta-C Plate System is designed to offer biomechanical stability in situ and flexibility in terms of implant range and configurations. The Mecta-C Anterior Cervical Plate System is designed to offer maximum biomechanical stability in situ and highflexibility in term of implants range selection and configurations.
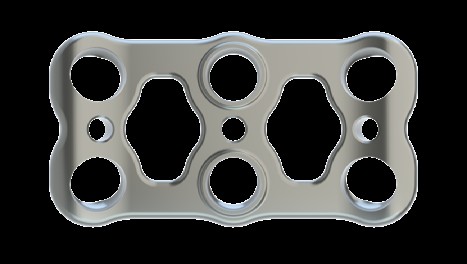


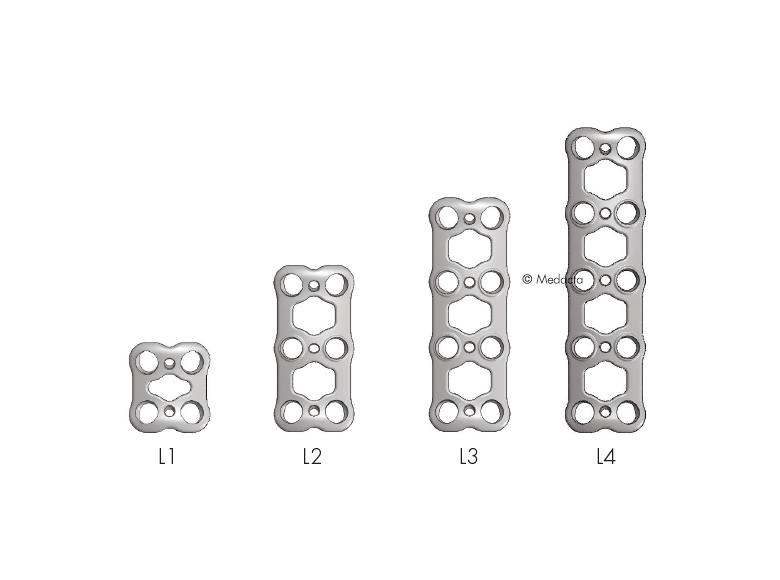


- 4 Plates: L1 - L4
- Pre-lordosed plates match the natural curvature of the spine
- Deliver secure fixation for one, two, three, and four level constructs
- Large central windows (9.5mm)
- Enhanced graft visibility
- Allow to center the plate to the cage
- Bone growth assessment
- Less than 2mm
- Reduce soft tissue irritation/dysphagia
- Reduce soft tissue irritation
- The Mecta-C Anterior Cervical Plate System devices are made of Titanium alloy (Ti-6Al-4V). The plates are prelordosed to match the natural curvature of the spine and are designed to cover up to four level configurations ranging from 20mm - 92mm in length. Also, constrained, semi-constrained or hybrid angle constructs may be built using Fixed or Variable angle bone screws system; provided with Self-Tapping and Self-Drilling options the bone screws are available in two diameters and several lengths. Overall, the different Mecta-C plates configurations bring beneficial advantages to meet different patients' needs in tumor, trauma and degenerative as well as in deformity cases.
- degenerative disc disease
- trauma
- tumors
- pseudarthrosis, and/or failed previous fusions
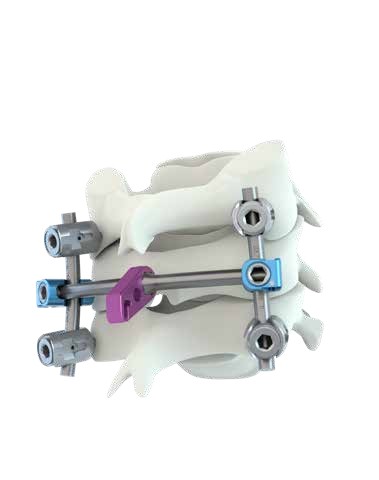
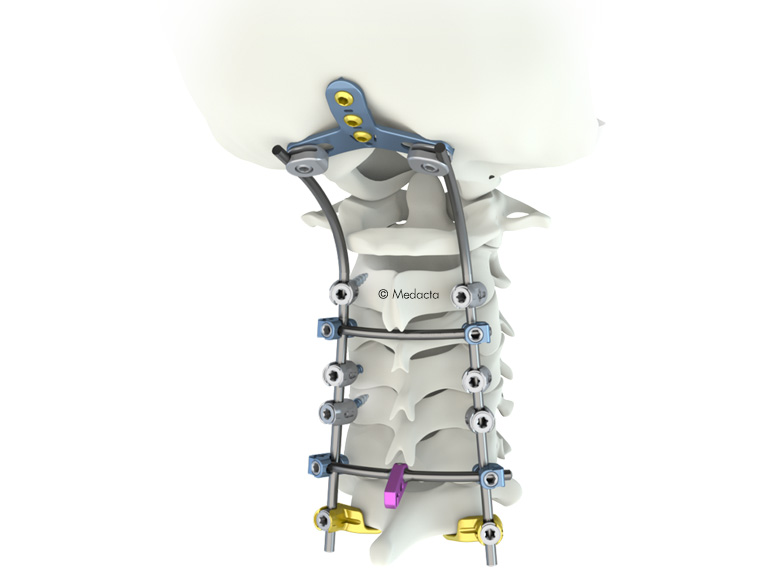
The M.U.S.T. MINI posterior cervical screw system is a comprehensive solution for fixation of the occipito-cervico-thoracicspine. The variety of screws, hooks, rods and connectors allows the surgeon to tailor the construct to the specific patient anatomy and pathology to be treated. Occipito-Cervical Fusion: The Occipital Plate offers flexibility to extend and further stabilise the posterior cervical construct. OC PLATES Small and large design to accommodate different patient anatomy. SCREW ALIGNMENT Screws aligned along the external protuberance to maximise bone purchase. SNAP-IN Adjustable OC Plate connectors with Snap-in feature to facilitate rod engagement. Unique Synergy With Myspine Cervical. SCREWS :Flat tip design with aggressive groove to facilitate screw insertion.







- Ccipito-Cervical Fusion
- Guided insertion of the M.U.S.T. MINI screws thanks to the MySpine Cervical, a 3D patient matched technology based on pre-operative planning designed for pedicle screw positioning
- The broad range of motion, up to 96° for the overall cone angle, eases the surgical practice in challenging anatomies.
- Comprehensive Solution
- All the M.U.S.T. MINI screws have the friction head feature to facilitate rod placement and ease of maneuvers during surgery.
- Fix and adapt the neck muscles in the midline
- Safely secure the bone graft with soft wires
- Protect in case of spinous process removal
- Naturally reshape the posterior aspect
- The system design is simple and flexible; a comprehensive set of components allows the surgeon to assemble the desired construct according to the anatomy of the patient and the pathology that requires treatment.
- The M.U.S.T. Mini posterior cervical screw system consists of polyaxial screws, three designs of occipital plate, hooks and multiple connectors
- The M.U.S.T. Mini posterior cervical screw system is intended to provide immobilization and stabilization of spinal segments as an adjunct to fusion for the following acute and chronic instabilities of the cervical spine and the thoracic spine from:
- traumatic spinal fractures, and/or traumatic dislocations, instability or deformity, failed previous fusions, tumors involving the cervical spine, degenerative disease, including intractable radiculopathy and/or myelopathy, neck and/or arm pain of discogenic origin as confirmed by radiographic studies, and degenerative disease of the facets with instability.
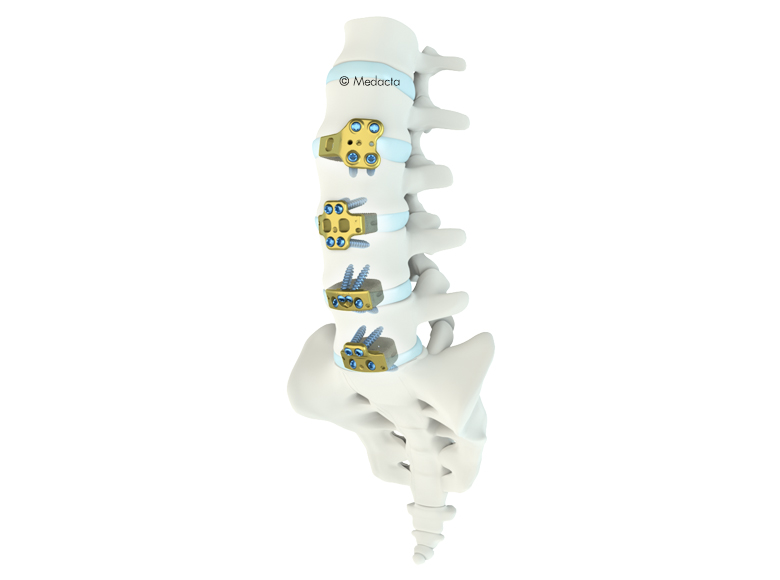
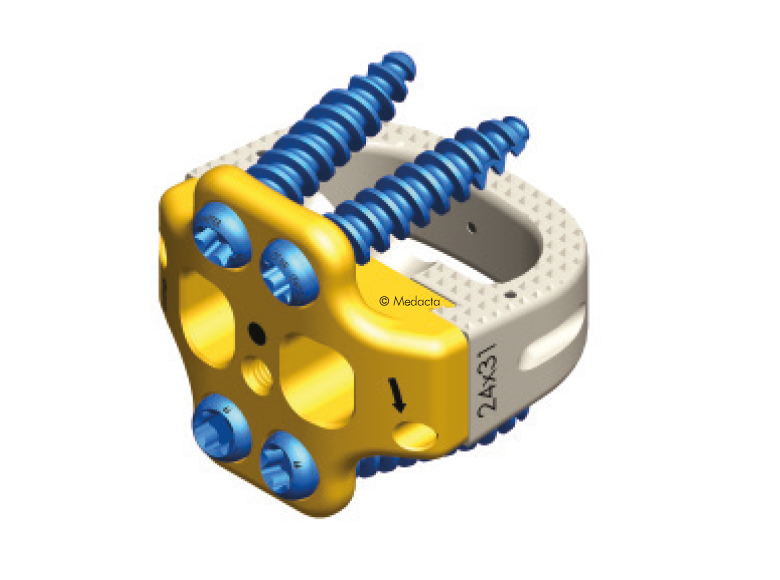
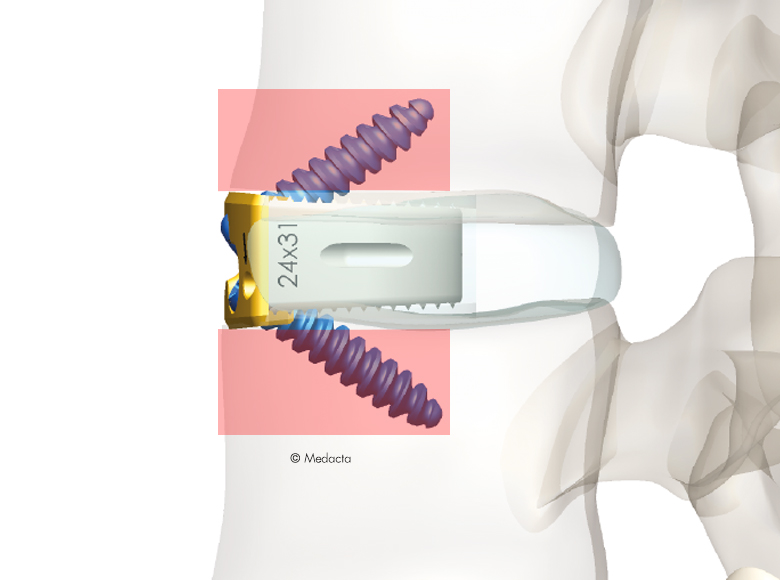
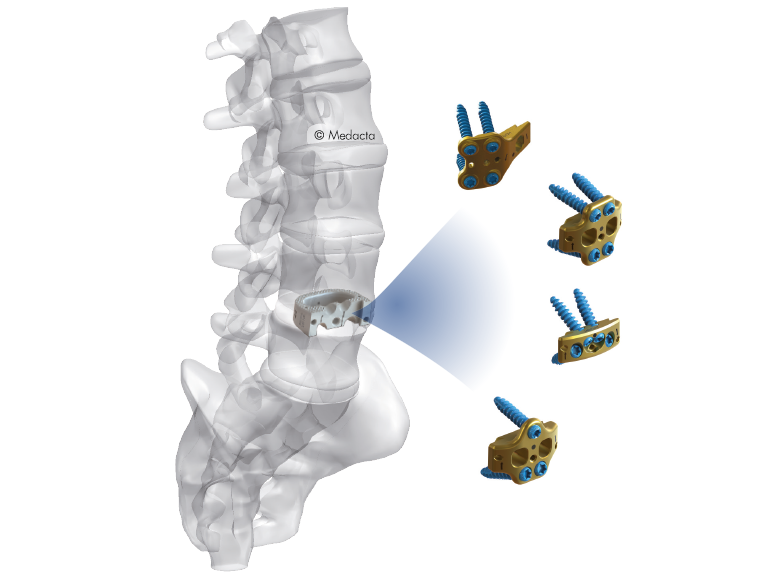
Mectalif anterior system, is a comprehensive solution for lumbar spine fusion. MectaLIF Anterior is a stand-alone spinal system specifically designed to provide your surgeon with multiple surgical options for correcting your spine. These fixation options are designed to offer the best solution to fit your spinal anatomy. The Anterior Lumbar Interbody Fusion surgery helps to stabilize your spine. The design incorporates the benefits of an anterior plate and a radiolucent interbody spacer. The five plate designs that can be used are designed to accomodate specific anatomic requirements. The system is completely modular. MectaLIF Anterior is a surgical solution specifically designed to provide multiple fixation options for patients undergoing anterior lumbar interbody fusion surgery. The cage is matched with a modular plate, selected among five different designs. Multiple screws are inserted through the holes of the plate into the vetebral body to fix the construct. It offers a cage with 3 different footprints, 4 degrees of lordosis and 5 heights, to which the surgeon can connect any of the four five profile plates that is required for the specific pathology the surgeon wants to treat. The MectaLIF Anterior is an interbody fusion device indicated for use in patients with degenerative disc disease at one or two contiguous levels

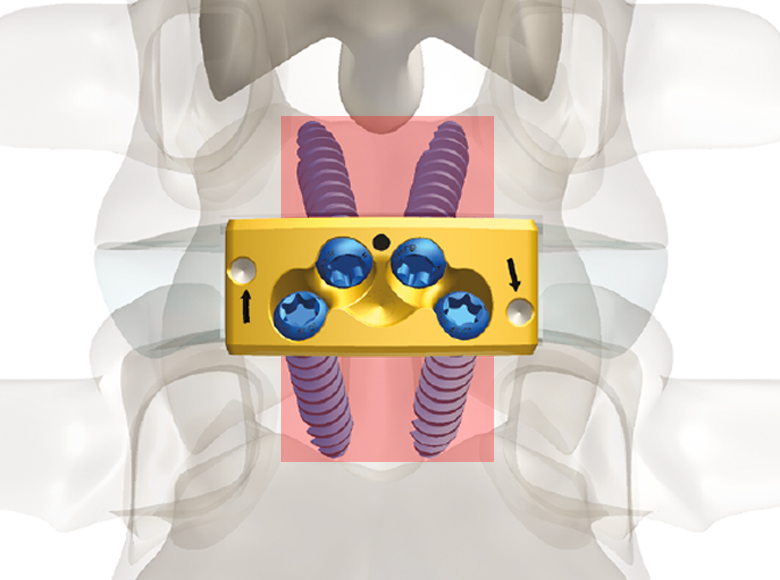
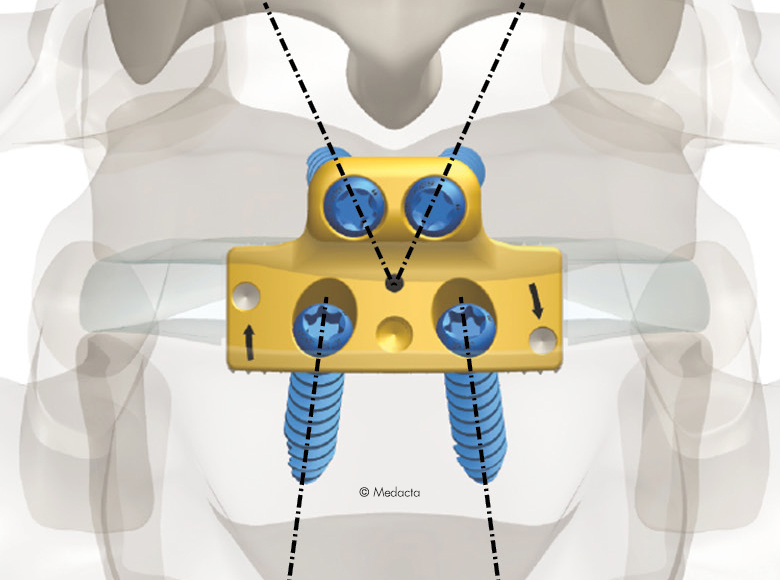

- Controlled torque means securely locked screws with no need for a separate anti-migration system
- Threaded Titanium helps avoid cross threading
- Horizontal screw angle reduces the bending moments, preventing screw back out
- Exclusive divergent & convergent screws
- Enhanced in-situ system stability
- Increased pull-out strength
- Large central bone graft area may help to accelerate the occurrence of fusion through the implant.
- The MectaLIF Anterior system exploits Medacta's uniquely bioactive and osteoconductive Titanium coating technology.
the MectalIF Family of Interbody Fusion devices are shaped for solid initial fixation, and long term spine stabilization. Made of PeeK and titanium coated PeeK material, the MectalIF Fusion devices offer biocompatibility and anatomic shaping to address your unique patients. The anatomical design of our MectaLIF Intervertebral Body Fusion Device matches the given biological conditions in each patient and pathology and meets the requirements of the treating surgeon. MectaLIF Posterior and MectaLIF Oblique Intervertebral Body Fusion Device, anatomical design features offers distinctive benefits: each implant features a chamfered leading edge for effective interbody distraction and less effort upon insertion. a variety of interbody heights, lordotic angles and footprints allow significant options when selecting the correct implant for your unique patients with bi-cortical bridging. large autograft windows allow the delivery of significant volumes of bone to support bone growth through the cages.
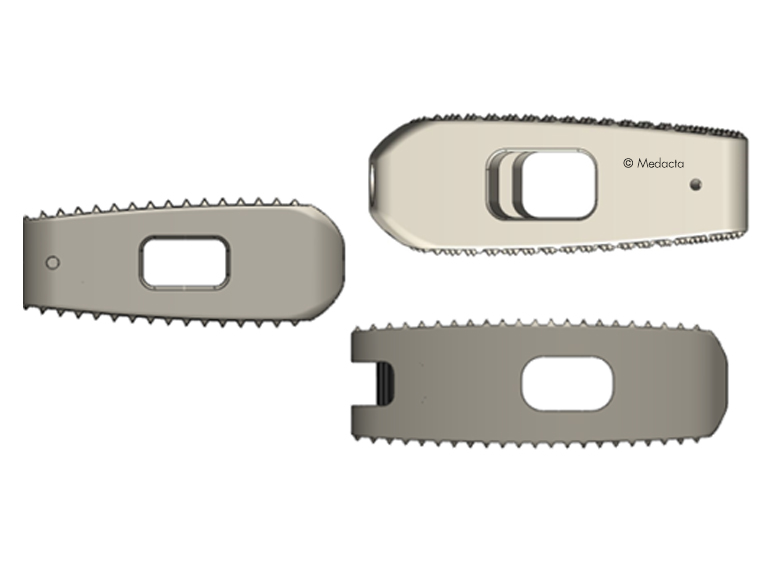
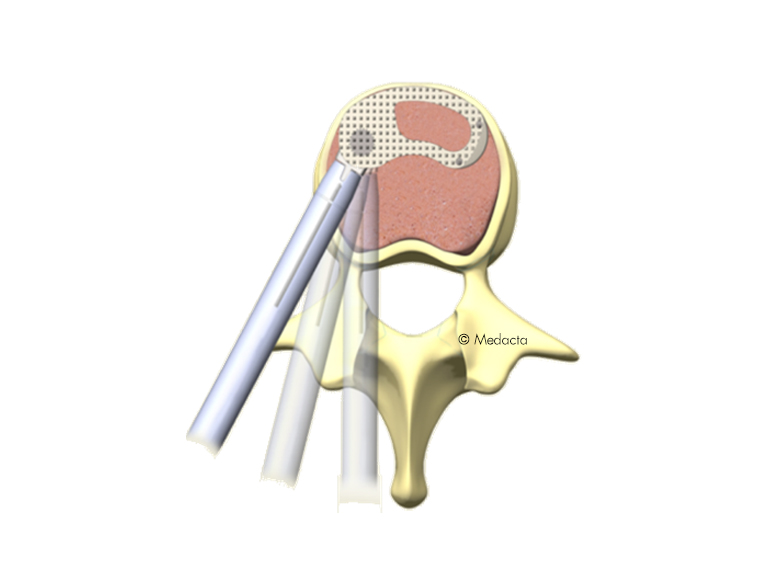


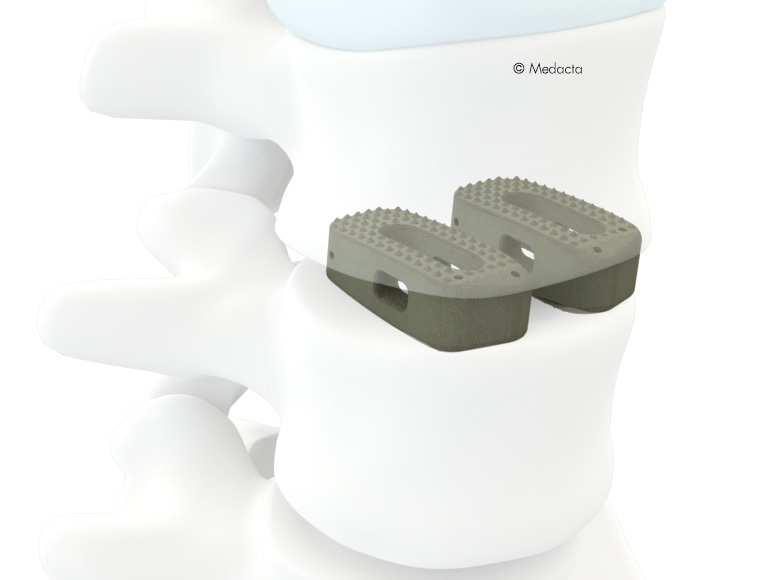

- Uniform, easy instrumentation for unilateral transforaminal/oblique approach (TLIF) or a bilateral posterior approach (PLIF)
- Biconvex superior/inferior surface that closely match the native anatomy
- Different footprints (three) and heights (nine) are offered to address individual patient anatomy
- The footprint as well as the outer counter is anatomically shaped to facilitate optimal load transfer and maximize the implant-endplate contact surface
- Large central as well as lateral window to receive filling material (bone graft or substitute) to accelerate the occurrence of fusion through the implant
- Pyramid shaped teeth to enhance both the implant stability and the resistance to implant migration
- Shapes ranging from parallel to lordotic to restore natural sagittal alignment
- Self-distracting bullet nose tip for simplicity of insertion
- Available in two versions: PEEK, TiPEEK
The Medacta Unconstrained Screw Technology [M.U.S.T.] Pedicle Screw System has been designed to give the surgeon ultimate flexibility in terms of choice of ideal bone anchor position, coupled with its unrivalled instrument handling capabilities that assist in spinal reduction, stabilization and ultimately fixation.. The M.U.S.T. system consists of a comprehensive range of devices to fully assist surgeons in the posterior spinal fixation. The M.U.S.T. Polyaxial Pedicle screw features a range of motion of greater than 60°, which coupled with dedicated instruments, allow the surgeon to achieve independent polyaxial tulip locking, allowing for easy parallel compression and distraction. These screws are available in a solid and a cannulated configuration giving the surgeons the chance to use them in standard open- as well as mini open surgeries. Furthermore, the broad range in size of the M.U.S.T. screws allows to cover primary as well as revision surgeries, completing the scenarios of application in the posterior spine pathology treatment. The M.U.S.T. Polyaxial Reduction Screw is designed to further complement the innovative design of the existing M.U.S.T. Polyaxial Screw range. These screws help to address, correct and also stabilize difficult anatomic variations. The Reduction Screw is designed with removable tabs that allow the surgeon to approximate the spine to the desired sagittal or axial profile. When used for posterior non-cervical pedicle screw fixation in pediatric patients, the M.U.S.T. implants are indicated as an adjunct to fusion to treat adolescent idiopathic scoliosis. The system is intended to be used with autograft and/or allograft. Pediatric applications are limited to a posterior approach.
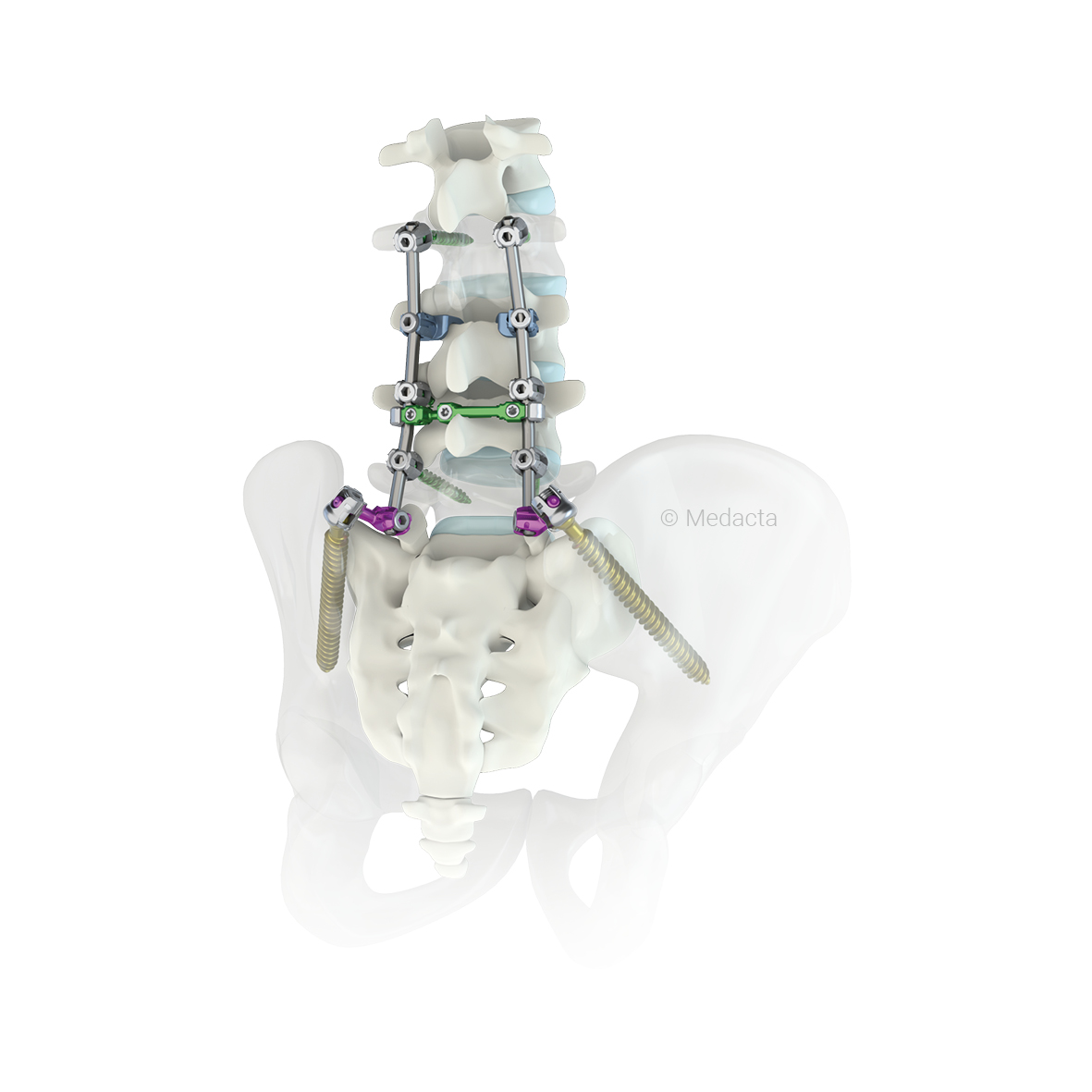
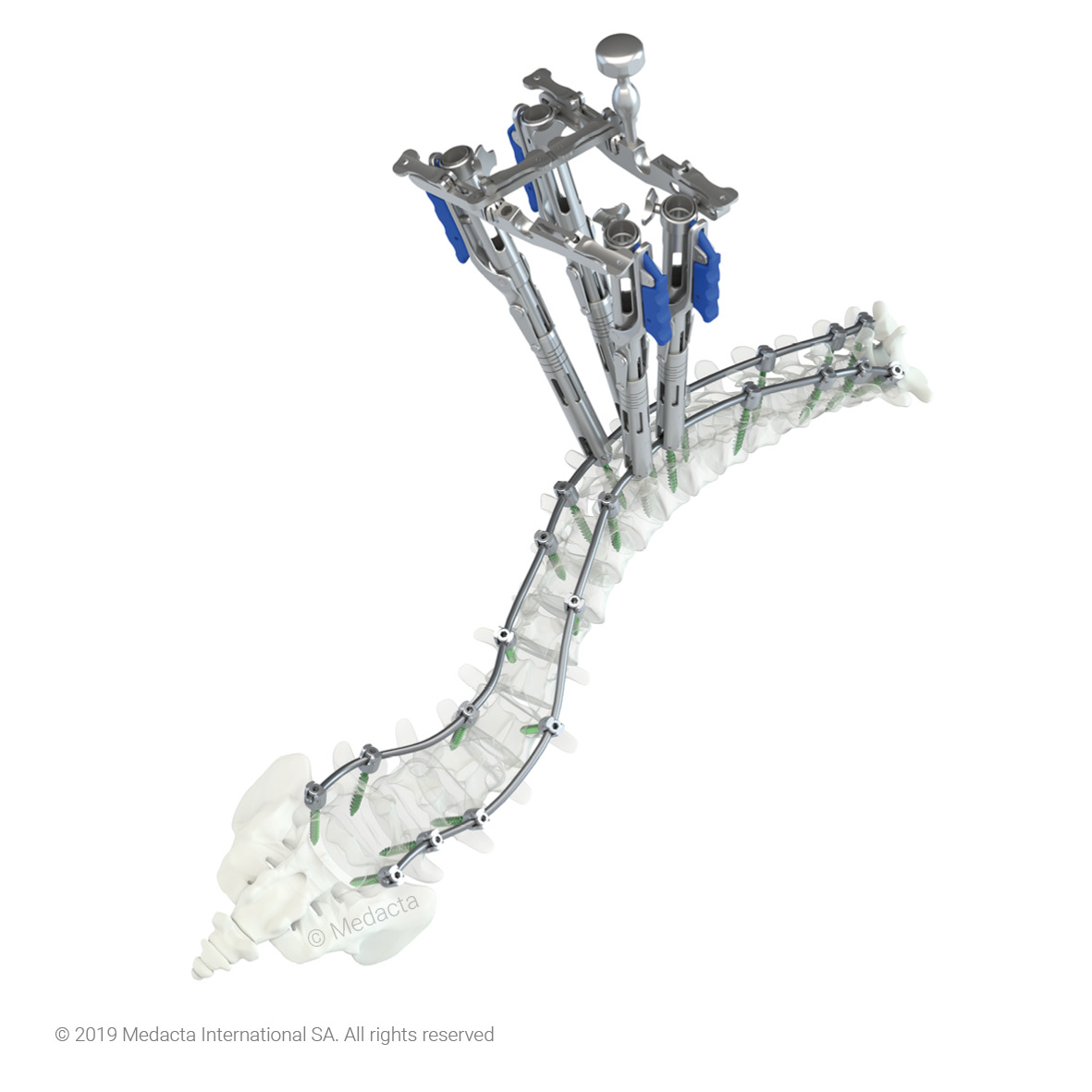
The Medacta M.U.S.T. LT system is a sub-family of the M.U.S.T. system designed to provide a minimally invasive solution for spinal thoracolumbar fixation. The system offers several advantages for patients including smaller incisions, minimal muscle resection, reduction of blood loss and post-operative pain, precision and efficiency in the pedicle screw placement. The M.U.S.T. LT implant is composed of the pedicle screw with welded tab, the set screw and the removable plastic sleeve. The M.U.S.T. LT system includes MIS Rods with bullet nose profile to ease the navigation through soft tissues while the hexagonal interface allows for the secure connection with the inserter.
- Comprehensive screw portfolio for thoracic and lumbar spine fixation
- Fenestrated screw option for cement-augmented fixation in case of osteoporotic bone
- Proper range of motion to fit several anatomical shapes (for all diameters)
- 100% Titanium alloy
- Pre-bent and straight rods to adapt to different anatomies
- 150mm tab length. Proper fit in multiple patient anatomies
- Cortical / cancellous threads differentiate bone purchases
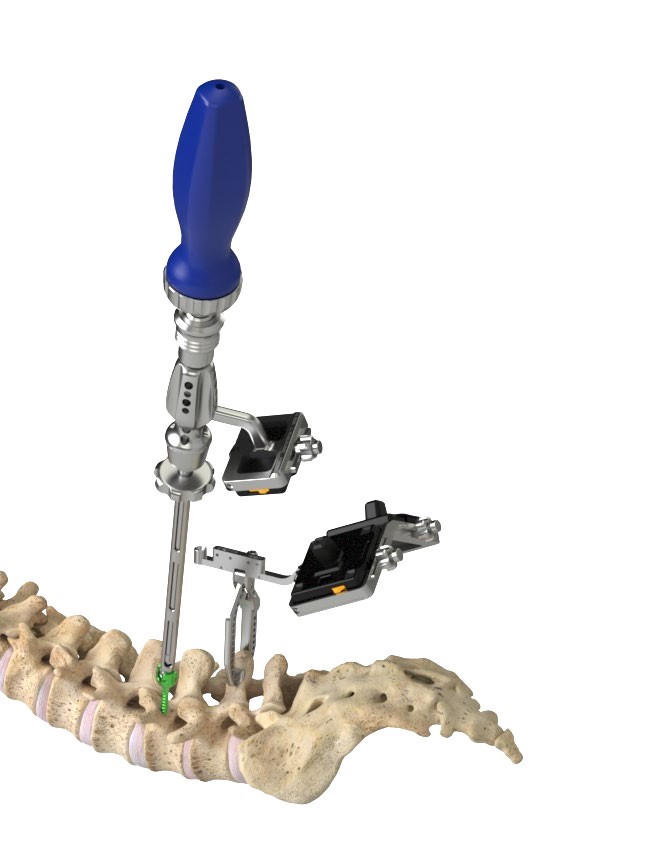
The Medacta MINI OPEN Retractor allows for decompression, fusion and fixation through a minimally invasive approach, however providing the surgeon the same versatility of an open procedure. The Medacta MINI OPEN Retractor represents a gentle interface to the soft tissues, potentially allowing to reduce blood loss and scarring, improve the patient's recovery time and shorten the hospital stay. The Medacta MINI OPEN Retractor allows for decompression, fusion and fixation through a Transforaminal, Wiltse approach. medacta international has designed a mini-open approach that utilizes our m.u.s.t. pedicle screw system and all the familiar instruments our Customers have endorsed…with a twist:
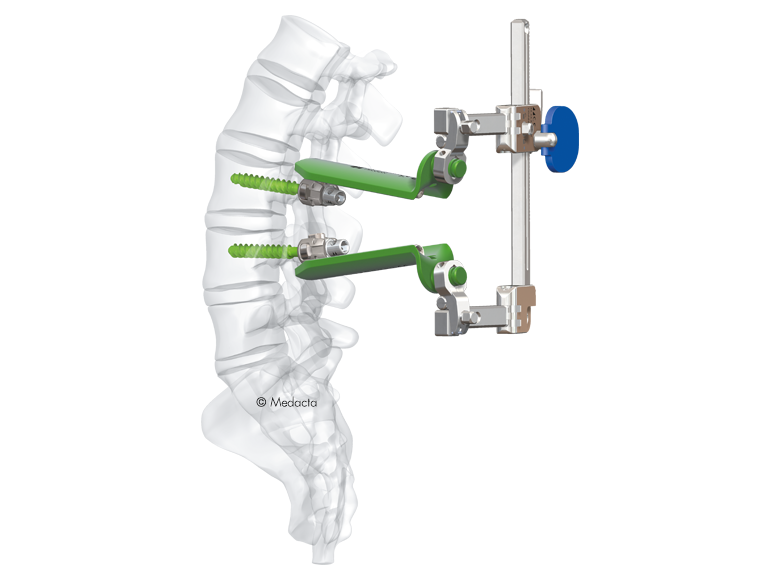
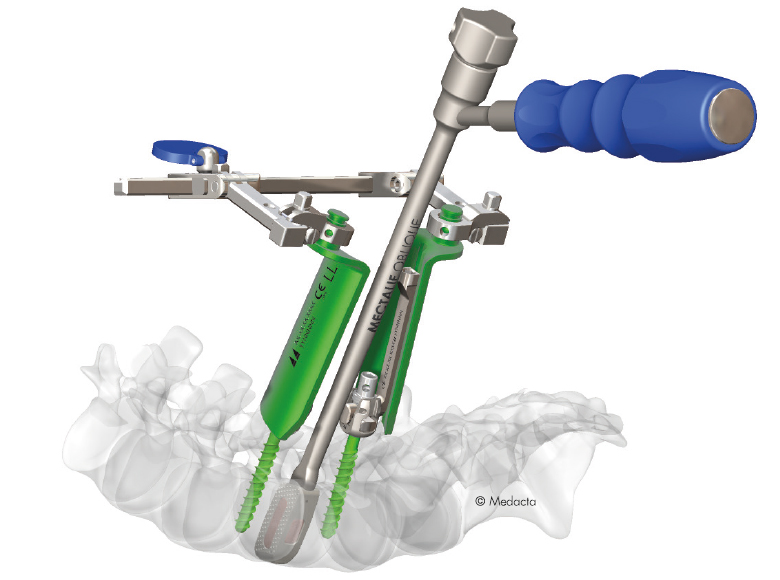
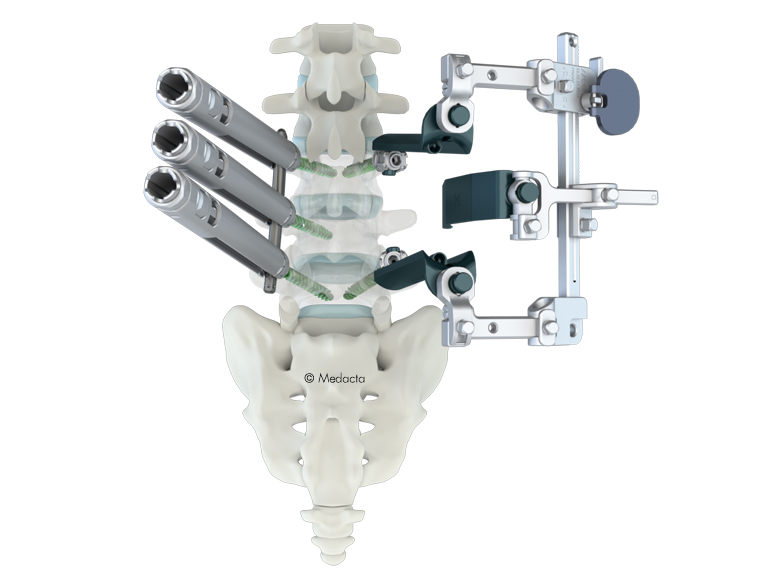
- option to provisionally lock the m.u.s.t. screw without a rod to perform parallel compression and distraction with our specially designed mini-open retractor as your mechanical advantage and control.
- Large visual in-situ exposure.
- Low profile parallel screw distraction.
- accommodates 1 and 2 level surgeries.
